the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Nitrate-dominated PM2.5 and elevation of particle pH observed in urban Beijing during the winter of 2017
Yuning Xie
Xinpei Wang
Jianmin Chen
Yubao Chen
Guiqian Tang
Lili Wang
Shuangshuang Ge
Guoyan Xue
Yuesi Wang
Jian Gao
The Chinese government has exerted strict emission controls to mitigate air pollution since 2013, which has resulted in significant decreases in the concentrations of air pollutants such as SO2. Strict pollution control actions also reduced the average PM2.5 concentration to the low level of 39.7 µg m−3 in urban Beijing during the winter of 2017. To investigate the impact of such changes on the physiochemical properties of atmospheric aerosols in China, we conducted a comprehensive observation focusing on PM2.5 in Beijing during the winter of 2017. Compared with the historical record (2014–2017), SO2 decreased to the low level of 3.2 ppbv in the winter of 2017, but the NO2 level was still high (21.4 ppbv in the winter of 2017). Accordingly, the contribution of nitrate (23.0 µg m−3) to PM2.5 far exceeded that of sulfate (13.1 µg m−3) during the pollution episodes, resulting in a significant increase in the nitrate-to-sulfate molar ratio. The thermodynamic model (ISORROPIA II) calculation results showed that during the PM2.5 pollution episodes particle pH increased from 4.4 (moderate acidic) to 5.4 (more neutralized) when the molar ratio of nitrate to sulfate increased from 1 to 5, indicating that aerosols were more neutralized as the nitrate content elevated. Controlled variable tests showed that the pH elevation should be attributed to nitrate fraction increase other than crustal ion and ammonia concentration increases. Based on the results of sensitivity tests, future prediction for the particle acidity change was discussed. We found that nitrate-rich particles in Beijing at low and moderate humid conditions (RH: 20 %–50 %) can absorb twice the amount of water that sulfate-rich particles can, and the nitrate and ammonia with higher levels have synergetic effects, rapidly elevating particle pH to merely neutral (above 5.6). As moderate haze events might occur more frequently under abundant ammonia and nitrate-dominated PM2.5 conditions, the major chemical processes during haze events and the control target should be re-evaluated to obtain the most effective control strategy.
- Article
(3809 KB) -
Supplement
(363 KB) - BibTeX
- EndNote
Severe haze pollution has been causing serious environmental problems and harming public health in China over the past decades (He et al., 2001; Wang et al., 2016; R. Zhang et al., 2015). Therefore, strong actions have been taken to improve the worsening atmospheric environment, including cutting down the pollutant emissions with forced installation of catalytic converters on vehicles, building clean-coal power generation systems, prohibiting open burning of crop residue during the harvest seasons, etc. (Chen et al., 2017; Zhang et al., 2012; Liu et al., 2016). As a result, the PM2.5 pollution occurrence has reduced to meet the goals in the Air Pollution Prevention and Control Action Plan (issued by the State Council of China, http://www.gov.cn/zwgk/2013-09/12/content_2486773.htm, last access: 24 February 2020, in Chinese). Among all the regions of interest, Beijing has achieved great success in PM2.5 reduction (the annual average PM2.5 concentration of 2017 was 58 µg m−3). Yet, PM2.5 concentration in Beijing is still higher than that in most developed countries.
There are many factors contributing to the PM2.5 pollution in China (Guo et al., 2014; Ding et al., 2013). The PM2.5 pollution across the country is characterized by significantly high secondary formation of inorganic components (R.-J. Huang et al., 2014). Sulfate, nitrate, and ammonium (SNA) comprised over 30 % of the PM2.5 mass, and SNA's fraction continues to increase during pollution evolution (Cao et al., 2012). While models can predict the airborne particle pollution in the US or Europe well, it is challenging to simulate the real atmospheric pollution in China (Wang et al., 2014; Ervens et al., 2003). Previous modeling studies showed that the simulated PM2.5 concentrations were underestimated within the current scheme, which is related to the important role of heterogeneous reactions in SNA formation processes (X. Huang et al., 2014; Herrmann et al., 2005). It was reported that the classical formation mechanism of sulfate in the atmosphere was through oxidation by H2O2. But recent studies in China pointed out that nonclassical formation pathways cannot be ignored. In Beijing, severe haze events occur with abundant nitrogen species (NOx, NH3, etc.), high relative humidity (RH), and less active photochemistry (Wang et al., 2016; Cheng et al., 2016). Field observations, chamber experiments, source apportionments, and numerical simulations all suggest that the joint effect of NO2, SO2, and NH3 is important for sulfate formation processes in haze events (Cheng et al., 2016; Wang et al., 2016; G. Wang et al., 2018; He et al., 2018, Xue et al., 2019). Aqueous oxidation of SO2 by NO2 as well as the catalyzed oxidation by transition metal ions (TMIs) could be major processes of sulfate formation in Beijing during winter (Wang et al., 2016; Cheng et al., 2016). In addition, although the photochemistry is less active during haze periods in winter, the extra OH radical provided by HONO might enhance the atmospheric oxidation capacity and lead to rapid formation of SNA (Tan et al., 2018, Ge et al., 2019). Since these reactions are all sensitive to particle acidity, adequate quantification of airborne particles' acidity is essential for elucidating the specific contribution.
Particle acidity has been widely studied due to its important role in haze formation and has been widely implemented in major models (Yu et al., 2005; Oleniacz et al., 2016). Since the practical method of directly measuring the particle acidity in the real atmosphere is not available (Wei et al., 2018; Freedman et al., 2019), thermodynamic models have been mostly used in quantifying the particle acidity. Most models (ISORROPIA II, E-AIM-IV, AIOMFAC, etc.) can predict H+, aerosol liquid water content (ALWC), and the partitioning of volatile and semi-volatile components, such as ammonia (Fountoukis and Nenes, 2007; Clegg et al., 2008). These models' abilities to describe physiochemical properties of airborne particles have been validated in previous studies (Weber et al., 2016; Guo et al., 2016; Shi et al., 2017; Tao and Murphy, 2019; Murphy et al., 2017). However, several publications using the same method gave different particle pH values in Beijing, and contradictory conclusions were drawn on the importance of sulfate formation by NO2 oxidation. Cheng et al. (2016) conducted some modeling work and suggested that the PM2.5 pH in Beijing ranges between 5.4 and 6.2, which is favorable for the aqueous NO2 oxidation. The NO2 oxidation's major contribution and the importance of high ALWC and sufficient ammonia are supported not only by modeling works, but also by field observation and chamber studies (Wang et al., 2016; Chen et al., 2019). Conversely, Liu et al. (2017) simulated the particle pH during the winter of 2015 and 2016 with the same method and claimed that pH of the Beijing haze particles was lower (3.0–4.9, average 4.2) and unfavorable for the NO2 oxidation mechanism. Based on the ISORROPIA II model results, which assumed Chinese haze particles to be a homogeneous inorganic mixture, Guo et al. (2017) further concluded that high ammonia cannot increase the particle pH enough for the aqueous oxidation of SO2 by NO2. Recently Song et al. (2018) reported that the thermodynamic model (ISORROPIA II) has coding errors, which can lead to the predicted pH values being negative or above 7. Furthermore, with lab studies and field observations, G. Wang et al. (2018) raised the concern of whether it is appropriate to elucidate sulfate production for Beijing haze by using particle pH predicted only based on the inorganic compositions. In fact, since the real atmosphere is affected by uncountable factors, it is common that particle pH has variation when simulated with the ambient data. Although the pH predicted by the thermodynamic models are of uncertainty, it is widely believed that haze particles in China are moderately acidic and are more neutralized than those in the US, given that gaseous ammonia is still at a high level relative to particulate ammonium (Song et al., 2018).
Air pollution control in China has entered the second phase – further mitigation of the moderate haze pollution, which is characterized by high levels of nitrate and ammonium and a low level of sulfate (Liu et al., 2019; de Foy et al., 2016) due to the efficient SO2 emission control. Such a change in chemical compositions could significantly alter physicochemical properties of the atmospheric aerosols in China. This paper aims to investigate the variation in the particle acidity of PM2.5 as sulfur emission is well controlled and nitrogen oxide emission remains high in Beijing. First, the compositions of air pollutants, including inorganic components of PM2.5 during the winter of 2017 and 2018, were analyzed and compared with previous studies; then, based on observations, the response of particle acidity to the elevation of nitrate was studied by using the ISORROPIA II thermodynamic model; finally, possible changes in the future are discussed based on the sensitivity tests.
The observations were conducted at an urban site – the State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute of Atmospheric Physics (IAP), Chinese Academy of Sciences ( N, E) in Beijing. All the instruments were on the roof of a two-story building. The local emissions are mainly from vehicles, and the industrial emissions are greatly reduced since the major factories and power plants have been moved out of Beijing or phased out due to the emission control policy. Overall, this site represents the typical atmospheric environment in urban Beijing, and the data obtained here can be compared with those from previous studies in the city (Ji et al., 2018).
A continuous online measurement of atmospheric components was conducted with a time resolution of 1 h. Two TEOM™ continuous ambient PM monitors using a PM2.5 or PM10 cyclone inlet (Met One) were applied to obtain PM2.5 and PM10 mass concentrations (Tang et al., 2016). For trace gases (O3, NO2, and SO2), a series of gas monitors were used for the hourly measurement (models 49i, 42i, and 43i, respectively) (Tang et al., 2012). Meteorology data, including ambient temperature, RH, wind speed, wind direction, and total solar radiation, were measured with an automatic weather station (MILOS520, Vaisala Inc., Finland) located in the middle of the observation site yard. Visibility data of Beijing were downloaded from the open database (https://gis.ncdc.noaa.gov/maps/ncei/cdo/hourly, last access: 27 July 2018). Apart from these online monitors, a high-volume sampler (Tisch Environmental) with a PM2.5 inlet was used to collect PM2.5 samples on a day–night basis (daytime at 08:00–17:50 and nighttime at 18:00–07:50).
The inorganic water-soluble components of PM2.5 (, , Cl−, NH4+, Na+, Ca2+, Mg2+, and K+) and ammonia gas were measured with an online-IC system: IGAC (in situ gas and aerosol composition monitor, Fortelice International Co., Ltd.). The IGAC is comprised of two parts: a sampling unit and an analyzer unit (Young et al., 2016). A vertical wet annular denuder (WAD) is used to collect the gas-phase species prior to a scrub and impactor aerosol collector (SIC), which can efficiently collect particles into liquid samples. During the campaign, 1 mM H2O2 solution is used as the absorption liquid for the air samples. Under most atmospheric conditions, the absorption liquid can efficiently absorb the target atmospheric components (e.g., SO2). An ICS-5000+ ion chromatograph is used as the analyzer unit in this study. For anions, an AS18 column (2 mm× 250 mm, Dionex™ IonPac™) is used while a CS-16 column (4 mm× 250 mm, Dionex™ IonPac™) is chosen to analyze major cations, both running with the recommended eluent (solution of KOH for anion and methane sulfonic acid for cation). The performance of the IGAC system has been tested and improved over recent years, and studies of PM2.5 water-soluble ion observations have been conducted by using it (Young et al., 2016; Song et al., 2018; Liu et al., 2017). A better sensitivity due to the advanced suppression technology of the system greatly enhances its ability to measure trace ions, such as sodium and magnesium, which is important in studies of particle ion balance. For details of the comparison between IGAC and filter sampling results, please refer to the Supplement (Fig. S1).
3.1 Major pollutants' levels
We first present the overall time series and statistics of major pollutants' concentration and meteorological parameters from 15 December 2017 to 25 February 2018. As shown in Fig. 1, during the observation campaign, Beijing was relatively cold and dry. Due to the frequent cold-air outbreaks, the average air temperature was around 0∘ with a minimum of −10∘, and the RH was low on average (20 %–30 %) with a maximum of 80 %. The average total solar radiation was 254.3 W m−2, which is typical in Beijing during winter. The wind usually blew from the north with an average speed of 1.9 m s−1, but strong wind (over 5 m s−1) frequently occurred on the clean days. Benefiting from the weather conditions, the atmospheric pollution in Beijing was much weaker than that in the winter of 2013. Overall, the improvement of the atmospheric environment was visible: the average visibility was around 15 km during the campaign and about 7.5 km during the pollution periods.
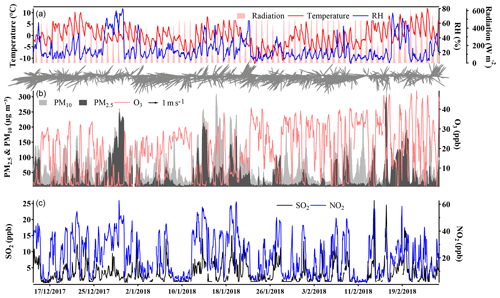
Figure 1Time series of major pollutants during the campaign. (a) Radiation, temperature RH, and wind arrows (drawn below); (b) PM2.5, PM10, and ozone concentration; (c) SO2 and NO2 concentrations.
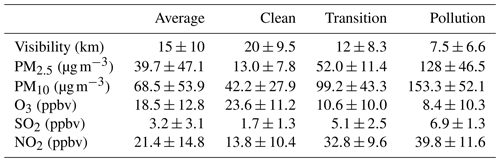
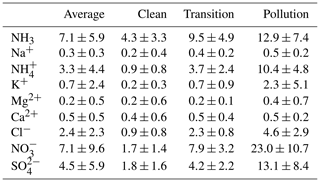
With strict control actions, there were fewer PM2.5 pollution episodes, and its concentration stayed at a low level most of the time in the winter of 2017. The average concentrations of PM2.5 and PM10 were 39.7 and 68.5 µg m−3, respectively. According to the PM2.5 concentration, three conditions of the atmospheric environment were classified in this study: clean (the PM2.5 was below 35 µg m−3), transition (the PM2.5 was between about 35 and 75 µg m−3), and pollution (the PM2.5 was above 75 µg m−3). In the clean, transition, and pollution periods, the average PM2.5 concentrations were 13.0±7.8, 52.0±11.4, and 128.0±46.5 µg m−3, respectively (as shown in Table 1), indicating that there was still PM2.5 pollution (maximum hourly concentration 298 µg m−3) during the winter. The average ozone concentration was 18.5±12.8 ppbv, and its value decreased as PM2.5 concentrations increased. The average SO2 concentration (3.2±3.1 ppbv) was almost 10 times lower than that of NO2 (21.4±14.8 ppbv). This significant contrast between SO2 and NO2 concentrations can be attributed to the sulfur emission controls over recent years and the fast increase in gasoline vehicles in Beijing (Cheng et al., 2018; T. Wang et al., 2018). All gaseous pollutants showed an increasing trend as the PM2.5 concentration increased during the haze episodes. While NO2 concentration elevation was the largest, NO2 and SO2 are the most important precursor gases for inorganic nitrate and sulfate in PM2.5. Sulfur emission control drastically reduced the ambient SO2 concentration while NO2 lacked effective control policy. To better describe this situation, changes in these two precursor gases during winter are investigated by examining the data from 2014 to 2016 in Beijing. Average values and the standard deviation are plotted in Fig. 2. SO2 showed a significant decreasing trend in all three conditions. In 2014, SO2 concentration was 3.9, 10.0, and 16.9 ppbv in clean, transition, and pollution periods, respectively. The SO2 concentration difference at different pollution levels was narrowing. Until 2017, the difference of SO2 concentrations between any two of the three conditions had all been within 10 ppbv. Meanwhile, NO2 concentrations kept increasing after 2015 in clean and transition conditions, but the NO2 concentration during the pollution periods of 2017 was unexpectedly lower than 2014 records. This significant drop of NO2 concentration in PM2.5 pollution proves the effectiveness of pollution control in 2017, including construction prohibition, private vehicle restriction, and liquefied natural gas (LNG) promotions in neighboring regions (Cheng et al., 2019).
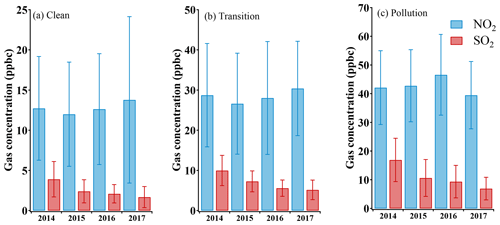
Figure 2Statistics plot of NO2 and SO2 measured in downtown Beijing at different PM2.5 levels during winter (December–February) for the past 4 years. Historical data (2014–2016) are from the air quality real-time publishing platform China National Environmental Monitoring Center, and data of 2017 have been obtained during the campaign.
3.2 PM2.5 chemical compositions
Compared to several previous reports, the chemical compositions of PM2.5 during the winter of 2017 in Beijing changed significantly (Shao et al., 2018; Elser et al., 2016; Ge et al., 2017; Huang et al., 2017; Wang et al., 2017). The major inorganic ions of PM2.5 in Beijing during the winter of 2017 included ammonium (3.3±4.4 µg m−3), nitrate (7.1±9.6 µg m−3), sulfate (4.5±5.9 µg m−3), and chloride (2.4±2.3 µg m−3). Concentrations of the major components increased as the PM2.5 concentration increased, but changes in the crustal ion (Na+, Mg2+, and Ca2+) concentrations were less significant. K+ increased during the PM2.5 pollution episodes (average concentration: 2.3±5.1 µg m−3), indicating the possible contribution of biomass burning sources or fireworks during the Chinese New Year. Cl− in PM2.5 has been used as a tracer for biomass burning and coal consumption. The concentration of Cl− (average concentration of 2.4±2.3 µg m−3) increased significantly as PM2.5 increased, but the imbalance of chloride molar concentration and potassium (average K+ of 0.059 µmol m−3 vs. average Cl− of 0.13 µmol m−3) suggests that biomass burning might not be the major source of PM2.5 chloride other than coal consumption during the PM2.5 pollution episodes in Beijing.
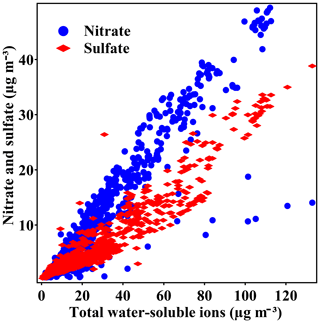
Concentration of sulfate, nitrate, and ammonium greatly increased the PM2.5 pollution. Different from previous records (Wang et al., 2016; R.-J. Huang et al., 2014; Ji et al., 2014), nitrate dominated the water-soluble ions (WSIs) in the winter of 2017. During pollution episodes, concentration of nitrate and sulfate were 23.0±10.7 and 13.1±8.4 µg m−3, with an average molar ratio of nitrate to sulfate (RatioN-to-S) of around 3.3±1.4. Figure 3 shows the scatter plot of nitrate and sulfate with the total water-soluble ions. Sulfate comprised a lower fraction when total WSIs were below 65 µg m−3, but the fraction increases as WSIs exceed 65 µg m−3, showing an enhanced formation of sulfate during heavy-pollution episodes. Interestingly, the ratio of nitrate to WSIs remained the same throughout the campaign. As the concentrations of other components also increased, this phenomenon indicated that the nitrate formation was enhanced on hazy days. In addition, the concentrations of ammonium and ammonia both increased significantly (ammonium: 0.9 to 10.4 µg m−3; ammonia: 4.3 to 12.9 µg m−3) from clean to pollution conditions.
3.3 Comparison of major inorganic compositions during the early 21st century in Beijing
To illustrate the changes in chemical compositions of PM2.5 during China's booming economy stage (1999–2017), nitrate, sulfate, and ammonium are chosen for the comparison with previously reported data during winter in Beijing (Fig. 4). Only winter-averaged observation data or representative pollution records are selected for the illustration on changes of secondary inorganic aerosols (SIAs) compositions. On average, although the concentration might have been varied due to different emissions and weather conditions over the years, SIA concentration in the winter of 2017 was the lowest compared with the years before. Sulfate concentration varied from 4.5 to 25.4 µg m−3 and contributed the most PM2.5 masses among SIA species during the pollution episodes before 2015. The emission control of SO2 started in 2006 to prevent adverse atmospheric environment events such as acid rain and high particulate matter loading (Wang et al., 2013; T. Wang et al., 2018). As a result, the sulfate concentration in winter decreased gradually (see results of 1999, 2011, 2015a, and 2017 shown in Fig. 4) until the record of recent years is much lower than that in the early 2000s (detailed literature comparison can be found in Lang et al., 2017). However, it was widely reported that sulfate still contributed the most to the PM2.5 mass concentration during the severe haze periods, such as during the winter of 2013 (R.-J. Huang et al., 2014; Guo et al., 2014; Ji et al., 2014). The heterogeneous formation might be responsible for the enhanced conversion ratio from SO2 to particulate sulfate, including the NO2-promoted aqueous reaction and transition-metal-catalyzed oxidations (X. Huang et al., 2014; Xie et al., 2015). On the other hand, the NOx emission in north China significantly increased as the power consumption and number of vehicles kept increasing. Therefore, nitrate in PM2.5 had been increasing since 2011. The average concentration of nitrate rose from 7.1 to 29.1 µg m−3. By 2015, the nitrate concentration had exceeded the sulfate concentration, and both compositions contributed equally to PM mass in winter pollution episodes. Although the nitrate concentration during pollution periods decreased in 2017 (23.0 µg m−3), the decrease was not significant and the concentration was still comparable to that in previous studies. The winter-averaged ammonium concentration reached the maximum (∼20 µg m−3) in 2015 but decreased afterwards. In a word, as the dominant composition, the high nitrate fraction has become one of the major features of PM2.5 in Beijing during winter.
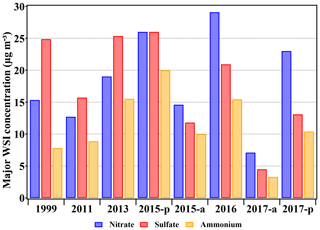
Figure 4Major inorganic compositions in PM2.5 observed in Beijing during winter from representative articles. The results of 2017 denote the average concentration during haze episodes in this study. For details of the reviewed literature, please refer to Table S1 in the Supplement.
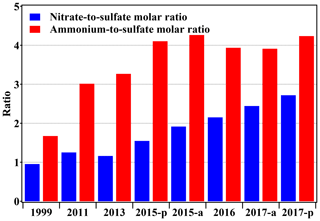
The ratio between major SIA components can better represent the composition change discussed above. As shown in Fig. 5, the nitrate-to-sulfate ratio (RatioN-to-S) has been increasing significantly from below 1.0 to 2.7 (1999 vs. 2017). RatioN-to-S was around 1 before 2013 but then steadily increased after 2013, the same as in previous publications (Shao et al., 2018; Lang et al., 2017). Interestingly, RatioN-to-S during pollution episodes was lower than the winter average value in 2015, but RatioN-to-S during pollution episodes greatly exceeded the average value in 2017, showing the dominance of nitrate in the PM2.5 pollution. The rapid increase in RatioN-to-S resulted not only from the sulfur emission control but also from more nitrate partitioning to the particle phase. Abundant ammonia in Beijing's atmosphere can enhance the partitioning of nitric acid gas by forming ammonium nitrate. To identify whether the ammonia is sufficient, the ammonium-to-sulfate ratio (RatioA-to-S) is calculated with the published data as well. It is reported that the North China Plain experienced ammonia insufficiency during summer (RatioA-to-S: less than 1.5), limiting the formation and partitioning of nitrate into the particle phase (Pathak et al., 2004, 2009, 2011). However, RatioA-to-S in Beijing during winter was always above 1.5. The lowest value appeared in 1999 (averaged RatioA-to-S: 1.7), and then the ratio increased rapidly (above 3) after 2011 (red bars in Fig. 5). In recent years, the RatioA-to-S has reached over 4. This value is typically observed in the eastern US during winter, though the absolute concentration is much higher in Beijing (Shah et al., 2018). To sum up, the effective sulfur emission control and ammonia-rich atmosphere provide a favorable environment for nitrate formation and eventually change PM2.5 in Beijing from sulfate-dominated to nitrate-dominated.
3.4 Aerosol pH's response to the elevation of nitrate fraction in PM2.5
The shift from sulfate-dominated to nitrate-dominated PM2.5 further influences the secondary chemical processes by changing physiochemical properties of aerosols, e.g., hygroscopicity and particle acidity. In a thorough study in the US, despite the good control of NOx emission, the nitrate fraction in PM2.5 did not show a corresponding decreasing trend. It was caused by the elevated partitioning of nitric acid to the particle phase in the eastern US (Shah et al., 2018). Researchers implied that higher nitrate partition fraction resulted from the increasing particle pH, while some studies showed that the particle pH decreases as particulate sulfate decreases in the US (Weber et al., 2016). To better understand the correlation between particle pH and chemical compositions, it is necessary to engage simulations with high-resolution observation datasets which cover as many pollution types as possible.
In this study, the bulk particle pH is calculated with the thermodynamic model ISORROPIA II in forward mode with the assumption of aerosol in metastable state. The simulation is limited to the data with the corresponding RH between 20 % and 90 %, the same as that in previous studies (Liu et al., 2017; Cheng et al., 2016). The analysis is further limited to data with sufficient ALWC (above 5 µg m−3) to avoid unrealistic pH values caused by false predictions of ALWC. To study the effect of the nitrate fraction's elevation on particle acidity, the RatioN-to-S is compared to the bulk particle pH (Fig. 6). As the nitrate fraction increases, the particle pH increases. When the RatioN-to-S is between 0 and 2, predicted pH values are rather scattered (2.1–6.2), with a median value of 4.4. As the ratio increases, pH values become less scattered and the median value increases as well. When the ratio is around 4–6, the predicted pH values range from 4.9 to 5.6 with a median value of 5.4, which is comparable with previous reported values in PM2.5 pollution episodes (Cheng et al., 2016; Wang et al., 2016; Xie et al., 2015). There are several possible explanations that could lead to pH increasing with higher RatioN-to-S, including neutralization by ammonia, higher pH of ammonium nitrate in comparison with ammonium sulfate, and increased ALWC leading to dilution of predicted H+ (Hodas et al., 2014; Xue et al., 2014; X. Wang et al., 2018). To confirm that the pH elevation is not caused by crustal ions, the simulation using data without crustal ions (input is set to 0) was conducted. It is shown that the exclusion of crustal ions in the simulation can cause an overall lower pH, but the pH elevation with RatioN-to-S is still observed (detailed analysis can be found in the Supplement, Figs. S2 and S3). On the other hand, as a major controlling factor (Guo et al., 2017; Song et al., 2018), ammonia concentration was even lower in the nitrate-dominated conditions (Fig. S4). In order to further elucidate the relationship between nitrate fraction and pH, a controlled sensitivity test was conducted for the Beijing PM2.5 aerosols by replacing particulate sulfate with the same moles of nitrate and keeping all other variables such as RH, temperature, ammonia, anions, and cations constant. As shown in Fig. 7, the median values of simulated pH increased from 4.6 to 5.1 as the transferring fraction increased from 10 % to 80 %. When the fraction exceeded 80 %, the median pH value was a bit lower compared to the pH80 % (Fig. 7a). By examining the difference between the sensitivity test results and the original pH values, an overall increase in pH by ∼0.5 was found when the fraction exceeds 60 % (Fig. 7b). The detailed sensitivity results and description also showed that the pH elevation due to the replacement of sulfate by nitrate was observed at all conditions (Fig. S5). With the fact that other variables remained controlled, the test results reconfirm that as nitrate becomes dominant, particle pH would significantly increase.
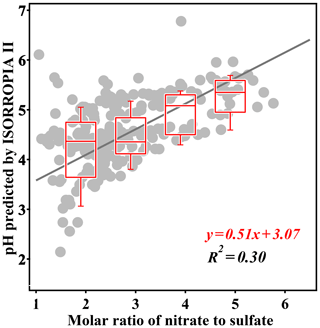
Figure 6Scatter plot of simulated pH vs. RatioN-to-S. Linear fitting and the correlation coefficient are given. The box plot denotes the data points classified by four different ranges of the RatioN-to-S: 0–2, 2–3, 3–4, and 4–6. Only the data corresponding to the pollution categories and with sufficient aerosol liquid water (above 5 µg m−3) are chosen, and the data during Chinese New Year are excluded.
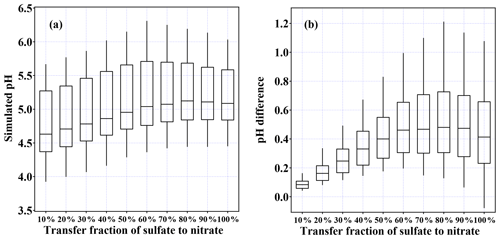
Figure 7Box plot of (a) simulated pH using the setting of the sensitivity test as well as (b) pH difference between the simulated pH from the transferred data and the original observation data (only data with sufficient aerosol liquid water content (above 5 µg m−3) during the pollution period were shown).
In this study, fewer predicted H+ ions in aerosol liquid water were found to be the major cause of the higher pH with a high nitrate fraction. The correlation between H+ and major anions (, , , Cl−) is shown in Fig. 8 to identify the acidity contribution of each anion. Sulfate and bisulfate have long been recognized as major acidic components of atmospheric particles. Their concentrations have significant impacts on the particle acidity (Weber et al., 2016; Liu et al., 2017). Therefore, the H+ ion concentration was found to strongly correlate with sulfate as well as bisulfate (Fig. 8a and b). The outlier data points can be attributed to the firework events during the Chinese New Year (extreme data on Chinese New Year's Eve are excluded). The average molar ratio of bisulfate to sulfate is , indicating that most of the sulfate is balanced by ammonium, the same as the results reported in previous studies (Song et al., 2018). The excess ammonium is then balanced by nitrate and chloride. The correlation between H+ and nitrate ions is much different as ALWC varies (Fig. 8c). Under the high-ALWC condition, the H+ increases with the nitrate concentration, which can be explained by the simultaneously increasing sulfate fraction during several pollution episodes. Under the drier condition (ALWC <10 µg m−3), as increases, H+ decreases, which implies that the weaker aerosol acidity favors nitric acid partitioning to the particle phase. Since HCl is more volatile than nitric acid gas, its occurrence in the particle phase is more sensitive to the particle acidity (Fig. 8d). Therefore, the negative correlation with H+ is much more obvious when it comes to chloride, independent of ALWC amount.
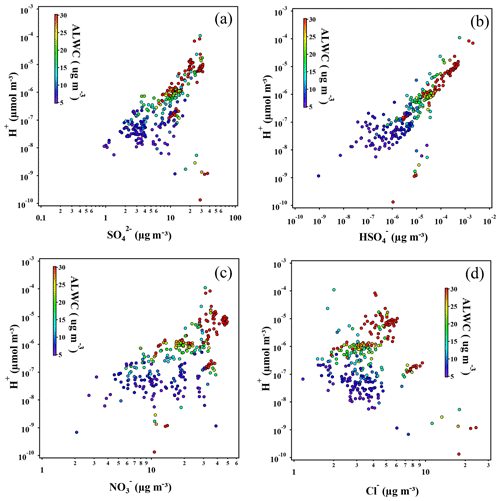
Figure 8Scatter plots of simulated H+ ion vs. major inorganic anions, including (a) , (b) , (c) , and (d) Cl−; the coordinates are logarithmic. Only the data corresponding to the pollution categories and with sufficient aerosol liquid water (above 5 µg m−3) were chosen, and the data during Chinese New Year are excluded.
The low level of H+, especially its negative correlation with RatioN-to-S, should be attributed to the neutralization by ammonia via gas-particle partitioning. Under most conditions, the excess of ammonia is an implicit prerequisite for SIA formation in Beijing, and higher NH3 concentration could increase the predicted particle pH (Guo et al., 2017; Weber et al., 2016). As auxiliary evidence, ammonia partition fraction (, calculated with observation data) exhibits a positive trend as RatioN-to-S increases (Fig. 9), while ammonia concentration remains less varied in the same case (Fig. S4). The positive trend is divided into two parts: a more acidic (pH below 4.5) branch with RatioN-to-S of 1–3 and of 0.1–0.6 and the other less acidic (pH above 5.5) branch with RatioN-to-S of 1–7 and of 0.1–0.4. The overall higher in the lower pH branch is reasonable since it is more favorable for ammonia partitioning to the particle phase when airborne particles exhibit higher acidity. Moreover, sulfate can accommodate twice the amount of ammonia than nitrate and thus increases . Yet, the highest values of were observed with more nitrate (RatioN-to-S∼2.5). By contrast, even though the high particle pH (5–6) may suppress ammonia from partitioning to the particle phase (Guo et al., 2017), elevation of with increasing RatioN-to-S (1–4) is still observed, with pH ranging from 5 to 6 despite there being some outliers with lower and lower NH3 concentration. Nitrate formation is observed to be enhanced in north China, either by heterogeneous formation (e.g., N2O5 hydrolysis) or with sufficient ambient NH3 (Wang et al., 2013). The positive trend of with RatioN-to-S clearly shows that nitrate formation and partitioning have a significant contribution to the NH3 partitioning process and will lead to an enhanced neutralization with the help of more ammonia partitioning into the particle phase. Combining these analyses, we conclude that the increasing nitrate fraction in fine particles will lead to higher particle pH in Beijing during winter.
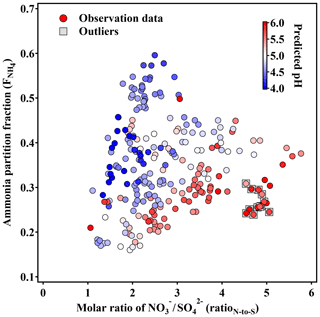
Figure 9Scatter plot of vs. the RatioN-to-S colored with predicted pH. Only the data corresponding to the pollution categories and with sufficient aerosol liquid water (above 5 µg m−3) were chosen, and the data during Chinese New Year are excluded. Note that the grey frame depicts the outliers which have lower and lower ammonia concentration.
So far, the effect of emission control on SNA compositions in Beijing's PM2.5 pollution and the response of particle pH have been illustrated, but it is important to make predictions with the current knowledge, providing scientific evidence for future better control strategy. In this section, sensitivity tests regarding hygroscopicity and particle-acidity change are conducted to help understand the possible changes of these properties in the future. ALWC is directly engaged in the calculation of particle pH and limited by several major parameters (RH, hydrophilic composition concentration, temperature, etc.). During the campaign, the ALWC predicted by ISORROPIA II varied between 0.8 and 154.2 µg m−3 with an average value of 6.4 µg m−3. As previously mentioned, the average value of RatioN-to-S is around 2 during the haze events in the winter of 2017 in Beijing. The average ALWC in the haze events increased to 24.4 µg m−3 accordingly. It has been reported that nitrate salts have a greater contribution to ALWC due to its lower deliquescence RH, and the elevated ALWC might have a strong impact on water-soluble gas partitioning such as that of glyoxal, leading to enhanced SOA production (Hodas et al., 2014; Xue et al., 2014). As a matter of fact, the increase in hygroscopicity related to nitrate-rich fine particles has been observed in Beijing (X. Wang et al., 2018). However, it is difficult to conclude that lower pH in nitrate-rich particles is caused by the dilution of H+ with higher ALWC with current data, since the higher nitrate fraction is usually observed with moderate RH in pollution episodes in the winter of 2017. Furthermore, the elevation of pH due to the RatioN-to-S increase is one unit of pH, which means the ALWC shall increase to 10 times the amount. This assumption is not supported by current data, since ALWC remains less varied as RatioN-to-S increases (Fig. S6). Similar to ALWC, the correlation between ambient temperature and RatioN-to-S is low, further proving that the increase in pH is not caused by change of thermodynamic state (Fig. S7).
The possible enhancement of hygroscopicity in nitrate-rich PM2.5 was investigated. Most single salts can only be deliquesced over a certain RH; thus the ALWC only exists when a certain RH is exceeded (Wexler and Seinfeld, 1991; Mauer and Taylor, 2010). In the real atmosphere, aerosols are usually a mixture of salts and organics, which might be easier to deliquesce. In addition, the deliquescence of NH4NO3 is unique, and it becomes more complicated in the system of NH4NO3∕(NH4)2SO4. The NH4NO3 system would absorb water vapor even at an extreme low RH, e.g., down to 10 % (Willeke et al., 1980; ten Brink and Veefkind, 1995). Previous studies show that the system comprised of NH4NO3∕(NH4)2SO4 has a higher deliquesce point when the sulfate content is higher (RatioN-to-S<1) but absorbs water even at low RH (∼20 %) when nitrate is dominant (RatioN-to-S>2) in PM2.5 inorganic ions (Wexler and Seinfeld, 1991; Ge et al., 1996, 1998). Inspired by these facts, we conducted a sensitivity test of ALWC to RH by using the observation dataset to study the effect of nitrate fraction elevation on ALWC changes (the RH value ranging from 20 % to 90 %, with 10 % as the interval). Concentrations of pollutants in the clean periods are relatively low and the data of the clean periods might be more influenced by the observation artifacts. Thus, only the data obtained in the transition and pollution conditions were analyzed here. The ALWC changes are defined as Eq. (1).
Then, we choose the data with RatioN-to-S above 3 and RatioN-to-S below 1. These values were both mentioned in previous lab studies (Ge et al., 1998) and are also typical values of nitrate-rich or sulfate-rich conditions in field observations. As shown in Fig. 10, PM2.5 with higher RatioN-to-S adsorbs more water than a lower nitrate fraction as the RH increases, which is more significant under lower RH (<50 %) conditions compared with that under higher RH (50 %–70 %) conditions. As the RH is usually lower (30 %–50) at the beginning stage of PM2.5 pollution development in Beijing, such a significant increase in hygroscopicity of nitrate-rich particles can greatly promote the haze formation under relatively dry conditions by enhancing the gas-to-particle partitioning of water-soluble compounds and the aqueous-phase formation of secondary aerosols, e.g., ammonia partitioning and nitrate formation through partitioning or hydrolysis of N2O5 (Badger et al., 2006; Bertram and Thornton 2009; Sun et al., 2018; Hodas et al., 2014; Shi et al., 2019).
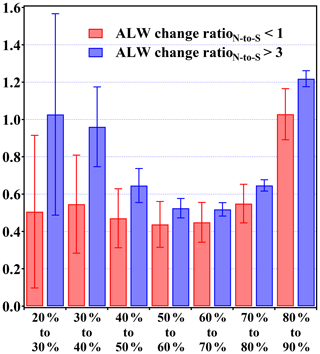
Figure 10Relative ALWC change due to the RH elevation at different RatioN-to-S. Bars represent the relative change amount, and whiskers depict the standard deviation.
The response of particle pH to ammonia and sulfate changes has been studied previously (Weber et al., 2016; Guo et al., 2017; Murphy et al., 2017). Here we further analyze the particle pH under the elevated nitrate concentration with the increasing ammonia in the atmosphere, which is the possible situation for most Chinese cities in the coming years. Two kinds of pH sensitivity tests are conducted: one with fixed nitrate but varying sulfate and ammonia and the other with fixed sulfate input but varying nitrate and ammonia (Fig. 11). In the test, crustal ions were all set as 0, while fixed chloride, sulfate, and nitrate concentrations were set as the average data on pollution (see Table 2). Compared with previous studies (Guo et al., 2017; Song et al., 2018), the RH was set as 58 % and the temperature was set as 273.15 K. Despite system errors due to the instability of the model at the extreme high-anion and low-NHx conditions (Song et al., 2018), the pH continuously changes as the free variable changes. The significant sharp edge of pH values in both plots defines the ion balance condition. We selected the observation data obtained during the pollution episodes within the RH ranging from 50 % to 70 % to compare with the results of both sensitivity tests. As shown in Fig. 11, apart from some data points (those with lower nitrate concentration but very high NHx concentration), observation data (triangles) are quite well merged into the results of sensitivity tests, and the pH values are generally higher than the test results due to the lack of crustal ion input in the sensitivity simulation. Therefore, the result of sensitivity tests can represent the pH change of the real atmosphere environment in Beijing well.
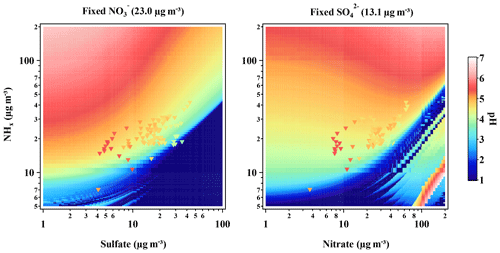
Figure 11Sensitivity tests of pH's response to sulfate or nitrate change with inputting the given NHx, simulated by ISORROPIA II. Field measurement data (triangles) were drawn upon the simulation data and colored with predicted pH, respectively. The simulation is conducted with fixed RH (58 %) and temperature (273.15 K).
Table 2Concentrations (µg m−3) of ammonia and inorganic ions of PM2.5 measured by IGAC during the campaign.
Future changes in particle pH can be found with the sensitivity test results. Cutting down the sulfate concentration without reducing atmospheric ammonia (horizontally moving from right to left in Fig. 10, left part) can lead to a significant increase in particle pH (up to 5). As can be seen from the right part of Fig. 10, the elevation of particle pH might be enhanced with the help of more nitrate in PM2.5. The effect of nitrate on particle pH greatly relies on the NHx concentration in the atmosphere. As the ammonia in the atmosphere over north China might still be increasing (Liu et al., 2018), and the sulfur content in the atmosphere might not be greatly reduced in the future, the particle pH shall increase in the path along the ion balance edge, which also implies a synergetic effect of increased nitrate and ammonia.
These results (lower acidity, higher hygroscopicity) provide insights into the effects of an elevated nitrate content on the physiochemical properties of particles. First, heterogeneous reactions that do not need high acidity might greatly contribute to the airborne particle chemistry, such as NO2-induced oxidation of the SO2 mechanism (Cheng et al. 2016; Wang et al., 2016). Reactions which rely greatly on acidified particles might contribute less, such as acid-catalyzed SOA formation from VOCs (Jang et al., 2002; Surratt et al., 2010). Second, the uptake processes of gaseous compounds onto particles (carbonyl acids, for example) might be enhanced, and the uptake of alkaline compounds could also be enhanced via the ALWC elevation. Third, optical properties of particles will greatly vary. On the one hand, higher ALWC can increase the light-scattering effect (Titos et al., 2014), while on the other hand the light absorption by BrC would be enhanced at higher pH (Phillips et al., 2017). All these facts might result in difficulties controlling moderate haze in Beijing, which usually occurs with lower RH and higher nitrate content as shown in this study. It is strongly suggested that the control strategy should be created accordingly based on thorough and scientific evaluation of both NOx and ammonia.
Due to strict emission controls, PM2.5 in Beijing during the winter of 2017 greatly decreased to a low level (39.7 µg m−3 for average concentration), but moderate haze episodes still frequently occurred in the city. With the observation and historical data, we found that the SO2 concentration decreased significantly while the NO2 concentration far exceeded that of SO2 and kept increasing in Beijing during winter. In response to the emission control, the nitrate concentration exceeded the concentration of sulfate significantly and thus became the dominant SIA component in fine particles. The molar ratio of nitrate to sulfate kept increasing over the years and rose to 2.7 during PM2.5 pollution episodes in the winter of 2017. The ammonium-to-sulfate ratio has always been above 1.5 in Beijing and has exceeded 3.0 since 2011. Sufficient ammonia provided strong atmospheric neutralization and weakened the particle acidity in Beijing, but the increased nitrate fraction was found to be causing the particle pH elevation. During the campaign, the pH of PM2.5 increased from 4.4 to 5.4 as the molar ratio of nitrate to sulfate increased from 1 to 5, which is firstly due to the lower amount of sulfate, which suppressed the formation of H+, and secondly due to the ammonia neutralization. Sensitivity tests of particle hygroscopicity and acidity were conducted to investigate the possible changes in physiochemical properties if ammonia and nitrate are not well controlled in China in the future. The results showed that the nitrate-rich particles can absorb more water than particles with higher sulfate fractions under moderate humid conditions (RH <60 %), and the particle pH increases rapidly due to the synergetic effect of ammonia and nitrate, which will very likely occur in China in the upcoming years, because both of these pollutants are not well controlled yet. The changes in particle pH and hygroscopicity will further enhance the uptake of gaseous compounds, promote chemical reactions which favor lower acidity, and also affect the optical properties of airborne particles in China. Therefore, the processes and properties of haze particles during nitrate-dominated periods in the country need to be thoroughly investigated with more consideration of highly hygroscopic and neutralized particles.
Data can be accessed by contacting the corresponding author.
The supplement related to this article is available online at: https://doi.org/10.5194/acp-20-5019-2020-supplement.
GW conceived the study and designed the experiment. YX conducted the online IGAC-PM2.5 chemical composition analysis and filter sampling in Beijing during the campaign. GT, LW, YW, and JG provided other related observation data used in this article, including trace gases, PM mass concentrations, and meteorological data. GW, XW, YC, GX, and SG conducted the lab analysis of filters and the data quality assurance and quality control. YX, GW, and JC performed the data analysis. YX and GW wrote the paper. All the co-authors contributed to the data interpretation and discussion.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Multiphase chemistry of secondary aerosol formation under severe haze”. It is not associated with a conference.
This work was financially supported by the National Key R&D Program of China (2017YFC0210000) and the National Nature Science Foundation of China (no. 41773117). We thank Yicheng Lin and Zhenrong Huang from Fortelice International Co., Ltd and Hanyu Gao from the Institute of Atmospheric Physics, Chinese Academy of Sciences for their technical support of IGAC operation during the campaign.
This research has been supported by the National Key R&D Program of China (grant no. 2017YFC0210000) and the National Nature Science Foundation of China (grant no. 41773117).
This paper was edited by Jingkun Jiang and reviewed by three anonymous referees.
Badger, C. L., Griffiths, P. T., George, I., Abbatt, J. P. D., and Cox, R. A.: Reactive Uptake of N2O5 by Aerosol Particles Containing Mixtures of Humic Acid and Ammonium Sulfate, J. Phys. Chem., 110, 6986–6994, https://doi.org/10.1021/jp0562678, 2006.
Bertram, T. H. and Thornton, J. A.: Toward a general parameterization of N2O5 reactivity on aqueous particles: the competing effects of particle liquid water, nitrate and chloride, Atmos. Chem. Phys., 9, 8351–8363, https://doi.org/10.5194/acp-9-8351-2009, 2009.
Cao, J.-J., Shen, Z.-X., Chow, J. C., Watson, J. G., Lee, S.-C., Tie, X.-X., Ho, K.-F., Wang, G.-H., and Han, Y.-M.: Winter and Summer PM2.5 Chemical Compositions in Fourteen Chinese Cities, J. Air. Waste. Manage., 62, 1214–1226, https://doi.org/10.1080/10962247.2012.701193, 2012.
Chen, J. M., Li, C. L., Ristovski, Z., Milic, A., Gu, Y. T., Islam, M. S., Wang, S. X., Hao, J. M., Zhang, H. F., He, C. R., Guo, H., Fu, H. B., Miljevic, B., Morawska, L., Thai, P., Fat, L., Pereira, G., Ding, A. J., Huang, X., and Dumka, U. C.: A review of biomass burning: Emissions and impacts on air quality, health and climate in China, Sci. Total Environ., 579, 1000–1034, https://doi.org/10.1016/j.scitotenv.2016.11.025, 2017.
Chen, T., Chu, B., Ge, Y., Zhang, S., Ma, Q., He, H., and Li, S.-M.: Enhancement of aqueous sulfate formation by the coexistence of NO2∕NH3 under high ionic strengths in aerosol water, Environ. Pollut., 252, 236–244, https://doi.org/10.1016/j.envpol.2019.05.119, 2019.
Cheng, J., Su, J., Cui, T., Li, X., Dong, X., Sun, F., Yang, Y., Tong, D., Zheng, Y., Li, Y., Li, J., Zhang, Q., and He, K.: Dominant role of emission reduction in PM2.5 air quality improvement in Beijing during 2013–2017: a model-based decomposition analysis, Atmos. Chem. Phys., 19, 6125–6146, https://doi.org/10.5194/acp-19-6125-2019, 2019.
Cheng, Y., Zheng, G., Wei, C., Mu, Q., Zheng, B., Wang, Z., Gao, M., Zhang, Q., He, K., Carmichael, G., Pöschl, U., and Su, H.: Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China, Sci. Adv., 2, e1601530, https://doi.org/10.1126/sciadv.1601530, 2016.
Clegg, S. L., Kleeman, M. J., Griffin, R. J., and Seinfeld, J. H.: Effects of uncertainties in the thermodynamic properties of aerosol components in an air quality model – Part 1: Treatment of inorganic electrolytes and organic compounds in the condensed phase, Atmos. Chem. Phys., 8, 1057–1085, https://doi.org/10.5194/acp-8-1057-2008, 2008.
de Foy, B., Lu, Z., and Streets, D. G.: Satellite NO2 retrievals suggest China has exceeded its NOx reduction goals from the twelfth Five-Year Plan, Sci. Rep., 6, 35912, https://doi.org/10.1038/srep35912, 2016.
Ding, A. J., Fu, C. B., Yang, X. Q., Sun, J. N., Zheng, L. F., Xie, Y. N., Herrmann, E., Nie, W., Petäjä, T., Kerminen, V.-M., and Kulmala, M.: Ozone and fine particle in the western Yangtze River Delta: an overview of 1 yr data at the SORPES station, Atmos. Chem. Phys., 13, 5813–5830, https://doi.org/10.5194/acp-13-5813-2013, 2013.
Elser, M., Huang, R.-J., Wolf, R., Slowik, J. G., Wang, Q., Canonaco, F., Li, G., Bozzetti, C., Daellenbach, K. R., Huang, Y., Zhang, R., Li, Z., Cao, J., Baltensperger, U., El-Haddad, I., and Prévôt, A. S. H.: New insights into PM2.5 chemical composition and sources in two major cities in China during extreme haze events using aerosol mass spectrometry, Atmos. Chem. Phys., 16, 3207–3225, https://doi.org/10.5194/acp-16-3207-2016, 2016.
Ervens, B., George, C., Williams, J. E., Buxton, G. V., Salmon, G. A., Bydder, M., Wilkinson, F., Dentener, F., Mirabel, P., Wolke, R., and Herrmann, H.: CAPRAM 2.4 (MODAC mechanism): An extended and condensed tropospheric aqueous phase mechanism and its application, J. Geophys. Res-Atmos., 108, 4426, https://doi.org/10.1029/2002JD002202, 2003.
Fountoukis, C. and Nenes, A.: ISORROPIA II: a computationally efficient thermodynamic equilibrium model for K+–Ca2+–Mg2+––Na+–––Cl−–H2O aerosols, Atmos. Chem. Phys., 7, 4639–4659, https://doi.org/10.5194/acp-7-4639-2007, 2007.
Freedman, M. A., Ott, E.-J. E., and Marak, K. E.: Role of pH in Aerosol Processes and Measurement Challenges, J. Phys. Chem., 123, 1275–1284, https://doi.org/10.1021/acs.jpca.8b10676, 2019.
Ge, S., Wang, G., Zhang, S., Li, D., Xie, Y., Wu, C., Yuan, Q., Chen, J., and Zhang, H.: Abundant NH3 in China Enhances Atmospheric HONO Production by Promoting the Heterogeneous Reaction of SO2 with NO2, Environ. Sci. Technol., 53, 14339–14347, https://doi.org/10.1021/acs.est.9b04196, 2019.
Ge, X., He, Y., Sun, Y., Xu, J., Wang, J., Shen, Y., and Chen, M.: Characteristics and Formation Mechanisms of Fine Particulate Nitrate in Typical Urban Areas in China, Atmosphere-Basel, 8, 62, https://doi.org/10.3390/atmos8030062, 2017.
Ge, Z., Wexler, A. S., and Johnston, M. V.: Multicomponent Aerosol Crystallization, J. Colloid. Interf. Sci., 183, 68–77, https://doi.org/10.1006/jcis.1996.0519, 1996.
Ge, Z., Wexler, A. S., and Johnston, M. V.: Deliquescence Behavior of Multicomponent Aerosols, J. Phys. Chem., 102, 173–180, https://doi.org/10.1021/jp972396f, 1998.
Guo, H., Sullivan, A. P., Campuzano-Jost, P., Schroder, J. C., Lopez-Hilfiker, F. D., Dibb, J. E., Jimenez, J. L., Thornton, J. A., Brown, S. S., Nenes, A., and Weber, R. J.: Fine particle pH and the partitioning of nitric acid during winter in the northeastern United States, J. Geophys. Res.-Atmos., 121, 10355–10376, https://doi.org/10.1002/2016JD025311, 2016.
Guo, H., Weber, R. J., and Nenes, A.: High levels of ammonia do not raise fine particle pH sufficiently to yield nitrogen oxide-dominated sulfate production, Sci. Rep., 7, 12109, https://doi.org/10.1038/s41598-017-11704-0, 2017.
Guo, S., Hu, M., Zamora, M. L., Peng, J., Shang, D., Zheng, J., Du, Z., Wu, Z., Shao, M., Zeng, L., Molina, M. J., and Zhang, R.: Elucidating severe urban haze formation in China, P. Natl. Acad. Sci. USA., 111, 17373, https://doi.org/10.1073/pnas.1419604111, 2014.
He, K., Yang, F., Ma, Y., Zhang, Q., Yao, X., Chan, C. K., Cadle, S., Chan, T., and Mulawa, P.: The characteristics of PM2.5 in Beijing, China, Atmos. Environ., 35, 4959-4970, https://doi.org/10.1016/S1352-2310(01)00301-6, 2001.
He, P., Alexander, B., Geng, L., Chi, X., Fan, S., Zhan, H., Kang, H., Zheng, G., Cheng, Y., Su, H., Liu, C., and Xie, Z.: Isotopic constraints on heterogeneous sulfate production in Beijing haze, Atmos. Chem. Phys., 18, 5515–5528, https://doi.org/10.5194/acp-18-5515-2018, 2018.
Herrmann, H., Tilgner, A., Barzaghi, P., Majdik, Z., Gligorovski, S., Poulain, L., and Monod, A.: Towards a more detailed description of tropospheric aqueous phase organic chemistry: CAPRAM 3.0, Atmos. Environ., 39, 4351–4363, https://doi.org/10.1016/j.atmosenv.2005.02.016, 2005.
Hodas, N., Sullivan, A. P., Skog, K., Keutsch, F. N., Collett, J. L., Decesari, S., Facchini, M. C., Carlton, A. G., Laaksonen, A., and Turpin, B. J.: Aerosol Liquid Water Driven by Anthropogenic Nitrate: Implications for Lifetimes of Water-Soluble Organic Gases and Potential for Secondary Organic Aerosol Formation, Environ. Sci. Technol., 48, 11127–11136, https://doi.org/10.1021/es5025096, 2014.
Huang, R.-J., Zhang, Y., Bozzetti, C., Ho, K.-F., Cao, J.-J., Han, Y., Daellenbach, K. R., Slowik, J. G., Platt, S. M., Canonaco, F., Zotter, P., Wolf, R., Pieber, S. M., Bruns, E. A., Crippa, M., Ciarelli, G., Piazzalunga, A., Schwikowski, M., Abbaszade, G., Schnelle-Kreis, J., Zimmermann, R., An, Z., Szidat, S., Baltensperger, U., Haddad, I. E., and Prévôt, A. S. H.: High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 514, 218–222, https://doi.org/10.1038/nature13774, 2014.
Huang, X., Song, Y., Zhao, C., Li, M., Zhu, T., Zhang, Q., and Zhang, X.: Pathways of sulfate enhancement by natural and anthropogenic mineral aerosols in China, J. Geophys. Res.-Atmos., 119, 14165–114179, https://doi.org/10.1002/2014JD022301, 2014.
Huang, X., Liu, Z., Liu, J., Hu, B., Wen, T., Tang, G., Zhang, J., Wu, F., Ji, D., Wang, L., and Wang, Y.: Chemical characterization and source identification of PM2.5 at multiple sites in the Beijing–Tianjin–Hebei region, China, Atmos. Chem. Phys., 17, 12941–12962, https://doi.org/10.5194/acp-17-12941-2017, 2017.
Jang, M., Czoschke, N. M., Lee, S., and Kamens, R. M.: Heterogeneous Atmospheric Aerosol Production by Acid-Catalyzed Particle-Phase Reactions, Science, 298, 814–817, https://doi.org/10.1126/science.1075798, 2002.
Ji, D., Li, L., Wang, Y., Zhang, J., Cheng, M., Sun, Y., Liu, Z., Wang, L., Tang, G., Hu, B., Chao, N., Wen, T., and Miao, H.: The heaviest particulate air-pollution episodes occurred in northern China in January, 2013: Insights gained from observation, Atmos. Environ., 92, 546–556, https://doi.org/10.1016/j.atmosenv.2014.04.048, 2014.
Ji, D., Cui, Y., Li, L., He, J., Wang, L., Zhang, H., Wang, W., Zhou, L., Maenhaut, W., Wen, T., and Wang, Y.: Characterization and source identification of fine particulate matter in urban Beijing during the 2015 Spring Festival, Sci. Total Environ., 628–629, 430–440, https://doi.org/10.1016/j.scitotenv.2018.01.304, 2018.
Lang, J., Zhang, Y., Zhou, Y., Cheng, S., Chen, D., Guo, X., Chen, S., Li, X., Xing, X., and Wang, H.: Trends of PM2.5 and Chemical Composition in Beijing, 2000–2015, Aerosol. Air. Qual. Res., 17, 412-425, https://doi.org/10.4209/aaqr.2016.07.0307, 2017.
Liu, F., Zhang, Q., van der A, R. J., Zheng, B., Tong, D., Yan, L., Zheng, Y., and He, K.: Recent reduction in NOx emissions over China: synthesis of satellite observations and emission inventories, Environ. Res. Lett., 11, 114002, https://doi.org/10.1088/1748-9326/11/11/114002, 2016.
Liu, M., Song, Y., Zhou, T., Xu, Z., Yan, C., Zheng, M., Wu, Z., Hu, M., Wu, Y., and Zhu, T.: Fine particle pH during severe haze episodes in northern China, Geophys. Res. Lett., 44, 5213–5221, https://doi.org/10.1002/2017GL073210, 2017.
Liu, M., Huang, X., Song, Y., Xu, T., Wang, S., Wu, Z., Hu, M., Zhang, L., Zhang, Q., Pan, Y., Liu, X., and Zhu, T.: Rapid SO2 emission reductions significantly increase tropospheric ammonia concentrations over the North China Plain, Atmos. Chem. Phys., 18, 17933–17943, https://doi.org/10.5194/acp-18-17933-2018, 2018.
Liu, M., Huang, X., Song, Y., Tang, J., Cao, J., Zhang, X., Zhang, Q., Wang, S., Xu, T., Kang, L., Cai, X., Zhang, H., Yang, F., Wang, H., Yu, J. Z., Lau, A. K. H., He, L., Huang, X., Duan, L., Ding, A., Xue, L., Gao, J., Liu, B., and Zhu, T.: Ammonia emission control in China would mitigate haze pollution and nitrogen deposition, but worsen acid rain, P. Natl. Acad. Sci. USA, 116, 7760, https://doi.org/10.1073/pnas.1814880116, 2019.
Mauer, L. J. and Taylor, L. S.: Water-Solids Interactions: Deliquescence, Annu. Rev. Food Sci. T., 1, 41–63, https://doi.org/10.1146/annurev.food.080708.100915, 2010.
Murphy, J. G., Gregoire, P. K., Tevlin, A. G., Wentworth, G. R., Ellis, R. A., Markovic, M. Z., and VandenBoer, T. C.: Observational constraints on particle acidity using measurements and modelling of particles and gases, Faraday Discuss., 200, 379–395, https://doi.org/10.1039/C7FD00086C, 2017.
Oleniacz, R., Rzeszutek, M., Bogacki, M.: Impact of Use of Chemical Transformation Modules in Calpuff on the Results of Air Dispersion Modelling, Ecol. Chem. Eng. S., 23, 605–620, https://doi.org/10.1515/eces-2016-0043, 2016.
Pathak, R. K., Yao, X., and Chan, C. K.: Sampling Artifacts of Acidity and Ionic Species in PM2.5, Environ. Sci. Technol., 38, 254–259, https://doi.org/10.1021/es0342244, 2004.
Pathak, R. K., Wu, W. S., and Wang, T.: Summertime PM2.5 ionic species in four major cities of China: nitrate formation in an ammonia-deficient atmosphere, Atmos. Chem. Phys., 9, 1711–1722, https://doi.org/10.5194/acp-9-1711-2009, 2009.
Pathak, R. K., Wang, T., and Wu, W. S.: Nighttime enhancement of PM2.5 nitrate in ammonia-poor atmospheric conditions in Beijing and Shanghai: Plausible contributions of heterogeneous hydrolysis of N2O5 and HNO3 partitioning, Atmos. Environ., 45, 1183–1191, https://doi.org/10.1016/j.atmosenv.2010.09.003, 2011.
Phillips, S. M., Bellcross, A. D., and Smith, G. D.: Light Absorption by Brown Carbon in the Southeastern United States is pH-dependent, Environ. Sci. Technol., 51, 6782–6790, https://doi.org/10.1021/acs.est.7b01116, 2017.
Shah, V., Jaeglé, L., Thornton, J. A., Lopez-Hilfiker, F. D., Lee, B. H., Schroder, J. C., Campuzano-Jost, P., Jimenez, J. L., Guo, H., Sullivan, A. P., Weber, R. J., Green, J. R., Fiddler, M. N., Bililign, S., Campos, T. L., Stell, M., Weinheimer, A. J., Montzka, D. D., and Brown, S. S.: Chemical feedbacks weaken the wintertime response of particulate sulfate and nitrate to emissions reductions over the eastern United States, P. Natl. Acad. Sci. USA, 115, 8110, https://doi.org/10.1073/pnas.1803295115, 2018.
Shao, P., Tian, H., Sun, Y., Liu, H., Wu, B., Liu, S., Liu, X., Wu, Y., Liang, W., Wang, Y., Gao, J., Xue, Y., Bai, X., Liu, W., Lin, S., and Hu, G.: Characterizing remarkable changes of severe haze events and chemical compositions in multi-size airborne particles (PM1, PM2.5 and PM10) from January 2013 to 2016–2017 winter in Beijing, China, Atmos. Environ., 189, 133–144, https://doi.org/10.1016/j.atmosenv.2018.06.038, 2018.
Shi, G., Xu, J., Peng, X., Xiao, Z., Chen, K., Tian, Y., Guan, X., Feng, Y., Yu, H., Nenes, A., and Russell, A. G.: pH of Aerosols in a Polluted Atmosphere: Source Contributions to Highly Acidic Aerosol, Environ. Sci. Technol., 51, 4289–4296, https://doi.org/10.1021/acs.est.6b05736, 2017.
Shi, X., Nenes, A., Xiao, Z., Song, S., Yu, H., Shi, G., Zhao, Q., Chen, K., Feng, Y., and Russell, A. G.: High-Resolution Data Sets Unravel the Effects of Sources and Meteorological Conditions on Nitrate and Its Gas-Particle Partitioning, Environ. Sci. Technol., 53, 3048–3057, https://doi.org/10.1021/acs.est.8b06524, 2019.
Song, S., Gao, M., Xu, W., Shao, J., Shi, G., Wang, S., Wang, Y., Sun, Y., and McElroy, M. B.: Fine-particle pH for Beijing winter haze as inferred from different thermodynamic equilibrium models, Atmos. Chem. Phys., 18, 7423–7438, https://doi.org/10.5194/acp-18-7423-2018, 2018.
Sun, P., Nie, W., Chi, X., Xie, Y., Huang, X., Xu, Z., Qi, X., Xu, Z., Wang, L., Wang, T., Zhang, Q., and Ding, A.: Two years of online measurement of fine particulate nitrate in the western Yangtze River Delta: influences of thermodynamics and N2O5 hydrolysis, Atmos. Chem. Phys., 18, 17177–17190, https://doi.org/10.5194/acp-18-17177-2018, 2018.
Surratt, J. D., Chan, A. W. H., Eddingsaas, N. C., Chan, M., Loza, C. L., Kwan, A. J., Hersey, S. P., Flagan, R. C., Wennberg, P. O., and Seinfeld, J. H.: Reactive intermediates revealed in secondary organic aerosol formation from isoprene, P. Natl. Acad. Sci. USA, 107, 6640, https://doi.org/10.1073/pnas.0911114107, 2010.
Tan, Z., Rohrer, F., Lu, K., Ma, X., Bohn, B., Broch, S., Dong, H., Fuchs, H., Gkatzelis, G. I., Hofzumahaus, A., Holland, F., Li, X., Liu, Y., Liu, Y., Novelli, A., Shao, M., Wang, H., Wu, Y., Zeng, L., Hu, M., Kiendler-Scharr, A., Wahner, A., and Zhang, Y.: Wintertime photochemistry in Beijing: observations of ROx radical concentrations in the North China Plain during the BEST-ONE campaign, Atmos. Chem. Phys., 18, 12391–12411, https://doi.org/10.5194/acp-18-12391-2018, 2018.
Tang, G., Wang, Y., Li, X., Ji, D., Hsu, S., and Gao, X.: Spatial-temporal variations in surface ozone in Northern China as observed during 2009–2010 and possible implications for future air quality control strategies, Atmos. Chem. Phys., 12, 2757–2776, https://doi.org/10.5194/acp-12-2757-2012, 2012.
Tang, G., Zhang, J., Zhu, X., Song, T., Münkel, C., Hu, B., Schäfer, K., Liu, Z., Zhang, J., Wang, L., Xin, J., Suppan, P., and Wang, Y.: Mixing layer height and its implications for air pollution over Beijing, China, Atmos. Chem. Phys., 16, 2459–2475, https://doi.org/10.5194/acp-16-2459-2016, 2016.
Tao, Y. and Murphy, J. G.: The sensitivity of PM2.5 acidity to meteorological parameters and chemical composition changes: 10-year records from six Canadian monitoring sites, Atmos. Chem. Phys., 19, 9309–9320, https://doi.org/10.5194/acp-19-9309-2019, 2019.
ten Brink, H. M. and Veefkind, J. P.: Humidity dependence of the light-scattering by ammonium nitrate, J. Aerosol. Sci., 26, S553–S554, https://doi.org/10.1016/0021-8502(95)97184-G, 1995.
Titos, G., Jefferson, A., Sheridan, P. J., Andrews, E., Lyamani, H., Alados-Arboledas, L., and Ogren, J. A.: Aerosol light-scattering enhancement due to water uptake during the TCAP campaign, Atmos. Chem. Phys., 14, 7031–7043, https://doi.org/10.5194/acp-14-7031-2014, 2014.
Wang, G., Zhang, R., Gomez, M. E., Yang, L., Levy Zamora, M., Hu, M., Lin, Y., Peng, J., Guo, S., Meng, J., Li, J., Cheng, C., Hu, T., Ren, Y., Wang, Y., Gao, J., Cao, J., An, Z., Zhou, W., Li, G., Wang, J., Tian, P., Marrero-Ortiz, W., Secrest, J., Du, Z., Zheng, J., Shang, D., Zeng, L., Shao, M., Wang, W., Huang, Y., Wang, Y., Zhu, Y., Li, Y., Hu, J., Pan, B., Cai, L., Cheng, Y., Ji, Y., Zhang, F., Rosenfeld, D., Liss, P. S., Duce, R. A., Kolb, C. E., and Molina, M. J.: Persistent sulfate formation from London Fog to Chinese haze, P. Natl. Acad. Sci. USA, 113, 13630, https://doi.org/10.1073/pnas.1616540113, 2016.
Wang, G., Zhang, F., Peng, J., Duan, L., Ji, Y., Marrero-Ortiz, W., Wang, J., Li, J., Wu, C., Cao, C., Wang, Y., Zheng, J., Secrest, J., Li, Y., Wang, Y., Li, H., Li, N., and Zhang, R.: Particle acidity and sulfate production during severe haze events in China cannot be reliably inferred by assuming a mixture of inorganic salts, Atmos. Chem. Phys., 18, 10123–10132, https://doi.org/10.5194/acp-18-10123-2018, 2018.
Wang, H., Lu, K., Chen, X., Zhu, Q., Chen, Q., Guo, S., Jiang, M., Li, X., Shang, D., Tan, Z., Wu, Y., Wu, Z., Zou, Q., Zheng, Y., Zeng, L., Zhu, T., Hu, M., and Zhang, Y.: High N2O5 Concentrations Observed in Urban Beijing: Implications of a Large Nitrate Formation Pathway, Environ. Sci. Tech. Let., 4, 416–420, https://doi.org/10.1021/acs.estlett.7b00341, 2017.
Wang, T., Wang, P., Theys, N., Tong, D., Hendrick, F., Zhang, Q., and Van Roozendael, M.: Spatial and temporal changes in SO2 regimes over China in the recent decade and the driving mechanism, Atmos. Chem. Phys., 18, 18063–18078, https://doi.org/10.5194/acp-18-18063-2018, 2018.
Wang, X., Shen, X. J., Sun, J. Y., Zhang, X. Y., Wang, Y. Q., Zhang, Y. M., Wang, P., Xia, C., Qi, X. F., and Zhong, J. T.: Size-resolved hygroscopic behavior of atmospheric aerosols during heavy aerosol pollution episodes in Beijing in December 2016, Atmos. Environ., 194, 188–197, https://doi.org/10.1016/j.atmosenv.2018.09.041, 2018.
Wang, Y., Zhang, Q. Q., He, K., Zhang, Q., and Chai, L.: Sulfate-nitrate-ammonium aerosols over China: response to 2000–2015 emission changes of sulfur dioxide, nitrogen oxides, and ammonia, Atmos. Chem. Phys., 13, 2635–2652, https://doi.org/10.5194/acp-13-2635-2013, 2013.
Wang, Y., Zhang, Q., Jiang, J., Zhou, W., Wang, B., He, K., Duan, F., Zhang, Q., Philip, S., and Xie, Y.: Enhanced sulfate formation during China's severe winter haze episode in January 2013 missing from current models, J. Geophys. Res.-Atmos., 119, 10425–410440, https://doi.org/10.1002/2013JD021426, 2014.
Weber, R. J., Guo, H., Russell, A. G., and Nenes, A.: High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years, Nat. Geosci., 9, 282–285, https://doi.org/10.1038/ngeo2665, 2016.
Wei, H., Vejerano, E. P., Leng, W., Huang, Q., Willner, M. R., Marr, L. C., and Vikesland, P. J.: Aerosol microdroplets exhibit a stable pH gradient, P. Natl. Acad. Sci. USA., 115, 7272, https://doi.org/10.1073/pnas.1720488115, 2018.
Wexler, A. S., and Seinfeld, J. H.: Second-generation inorganic aerosol model, Atmos. Environ. A-Gen., 25, 2731–2748, https://doi.org/10.1016/0960-1686(91)90203-J, 1991.
Willeke, K., Society, A. C., and Kagakkai, N.: Generation of Aerosols and Facilities for Exposure Experiments, edited by: Willeke, K., Ann Arbor Science Publishers Inc. Michigan, 1980.
Xie, Y., Ding, A., Nie, W., Mao, H., Qi, X., Huang, X., Xu, Z., Kerminen, V.-M., Petäjä, T., Chi, X., Virkkula, A., Boy, M., Xue, L., Guo, J., Sun, J., Yang, X., Kulmala, M., and Fu, C.: Enhanced sulfate formation by nitrogen dioxide: Implications from in situ observations at the SORPES station, J. Geophys. Res.-Atmos., 120, 12679–12694, https://doi.org/10.1002/2015JD023607, 2015.
Xue, J., Griffith, S. M., Yu, X., Lau, A. K. H., and Yu, J. Z.: Effect of nitrate and sulfate relative abundance in PM2.5 on liquid water content explored through half-hourly observations of inorganic soluble aerosols at a polluted receptor site, Atmos. Environ., 99, 24–31, https://doi.org/10.1016/j.atmosenv.2014.09.049, 2014.
Xue, J., Yu, X., Yuan, Z., Griffith, S. M., Lau, A. K. H., Seinfeld, J. H., and Yu, J. Z.: Efficient control of atmospheric sulfate production based on three formation regimes, Nat. Geosci., 12, 977–982, https://doi.org/10.1038/s41561-019-0485-5, 2019.
Young, L.-H., Li, C.-H., Lin, M.-Y., Hwang, B.-F., Hsu, H.-T., Chen, Y.-C., Jung, C.-R., Chen, K.-C., Cheng, D.-H., Wang, V.-S., Chiang, H.-C., and Tsai, P.-J.: Field performance of a semi-continuous monitor for ambient PM2.5 water-soluble inorganic ions and gases at a suburban site, Atmos. Environ., 144, 376–388, https://doi.org/10.1016/j.atmosenv.2016.08.062, 2016.
Yu, S., Dennis, R., Roselle, S., Nenes, A., Walker, J., Eder, B., Schere, K., Swall, J., and Robarge, W.: An assessment of the ability of three-dimensional air quality models with current thermodynamic equilibrium models to predict aerosol , J. Geophys. Res.-Atmos., 110, D07S13, https://doi.org/10.1029/2004JD004718, 2005.
Zhang, Q., He, K., and Huo, H.: Cleaning China's air, Nature, 484, 161–162, https://doi.org/10.1038/484161a, 2012.
Zhang, Q., Quan, J. N., Tie, X. X., Li, X., Liu, Q., Gao, Y., and Zhao, D. L.: Effects of meteorology and secondary particle formation on visibility during heavy haze events in Beijing, China, Sci. Total Environ., 502, 578–584, https://doi.org/10.1016/j.scitotenv.2014.09.079, 2015a.
Zhang, R., Wang, G., Guo, S., Zamora, M. L., Ying, Q., Lin, Y., Wang, W., Hu, M., and Wang, Y.: Formation of Urban Fine Particulate Matter, Chem. Rev., 115, 3803–3855, https://doi.org/10.1021/acs.chemrev.5b00067, 2015b.
- Abstract
- Introduction
- Sampling site and instrumentation
- Results
- Discussion: possible impacts of increasing fraction of nitrate in PM2.5
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Sampling site and instrumentation
- Results
- Discussion: possible impacts of increasing fraction of nitrate in PM2.5
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement