the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
High secondary formation of nitrogen-containing organics (NOCs) and its possible link to oxidized organics and ammonium
Guohua Zhang
Xiufeng Lian
Yuzhen Fu
Qinhao Lin
Wei Song
Zhanyong Wang
Mingjin Tang
Duohong Chen
Xinming Wang
Guoying Sheng
Nitrogen-containing organic compounds (NOCs) substantially contribute to light-absorbing organic aerosols, although the atmospheric processes responsible for the secondary formation of these compounds are poorly understood. In this study, seasonal atmospheric processing of NOCs is investigated using single-particle mass spectrometry in urban Guangzhou from 2013 to 2014. The relative abundance of NOCs is found to be strongly enhanced when they are internally mixed with photochemically produced secondary oxidized organics (i.e., formate, acetate, pyruvate, methylglyoxal, glyoxylate, oxalate, malonate, and succinate) and ammonium (). Moreover, both the hourly detected particle number and the relative abundance of NOCs are highly correlated with those of secondary oxidized organics and . Therefore, it is hypothesized that the secondary formation of NOCs is most likely linked to oxidized organics and . Results from both multiple linear regression analysis and positive matrix factorization analysis further show that the relative abundance of NOCs could be well predicted (R2 > 0.7, p < 0.01) by oxidized organics and .
Interestingly, the relative abundance of NOCs is inversely correlated with
, whereas their number fractions are positively correlated. This
result suggests that although the formation of NOCs does require the
involvement of , the relative amount of may have
a negative effect. Higher humidity and NOx likely facilitates the conversion
of oxidized organics to NOCs. Due to the relatively high oxidized organics
and , the relative contributions of NOCs in summer and
fall were higher than those in spring and winter. To the best of our
knowledge, this is the first direct field observation study reporting a
close association between NOCs and both oxidized organics and .
These findings have substantial implications for the role of in the
atmosphere, particularly in models that predict the evolution and deposition
of NOCs.
Highlights.
-
NOCs were highly internally mixed with photochemically produced secondary oxidized organics
-
NOCs could be well predicted by the variations of these oxidized organics and
-
Higher relative humidity and NOx may facilitate the conversion of these oxidized organics to NOCs
- Article
(2243 KB) -
Supplement
(777 KB) - BibTeX
- EndNote
Organic aerosols that strongly absorb solar radiation are referred to as brown carbon (BrC). BrC has a comparable level of light absorption in the spectral range of near-ultraviolet (UV) light to black carbon (Andreae and Gelencser, 2006; Feng et al., 2013; Yan et al., 2018). Nitrogen-containing organic compounds (NOCs) substantially contribute to the pool of BrC (Mohr et al., 2013; Li et al., 2019) and have a significant effect on atmospheric chemistry, human health, and climate forcing (Kanakidou et al., 2005; Shrivastava et al., 2017; De Gouw and Jimenez, 2009). Particulate organic nitrogen accounts for a large fraction of total airborne nitrogen (∼30 %), although the proportion exhibits a high temporal and spacial variability and, therefore, has an influence on both regional and global nitrogen (N) deposition (Neff et al., 2002; Shi et al., 2010; Cape et al., 2011). However, the sources, evolution, and optical properties of NOCs remain unclear and contribute significantly to uncertainties in the estimation of their impacts on the environment and climate (Laskin et al., 2015).
NOCs are ubiquitous components in atmospheric aerosols, cloud water, and rainwater (Altieri et al., 2009; Desyaterik et al., 2013; Laskin et al., 2015), spanning a wide range of molecular weights, structures, and light absorption properties (Lin et al., 2016). Emissions of primary NOCs have been attributed to biomass burning, coal combustion, vehicle emissions, biogenic production, and soil dust (Laskin et al., 2009; Desyaterik et al., 2013; Sun et al., 2017; Mace et al., 2003; Rastogi et al., 2011; Wang et al., 2017). Secondary NOCs, such as organic nitrates and nitroaromatic compounds, are believed to be mainly formed in the gas phase by interaction between volatile organic compounds (VOCs) and oxidants (e.g., NOx, • OH), followed by condensation to aerosols (Ziemann and Atkinson, 2012; Seinfeld and Pandis, 2006). Recently, another group of secondary NOCs, or heterocyclic NOCs, formed by reactions involving mixtures of atmospheric aldehydes (e.g., methylglyoxal/glyoxal) and ammonium ()/amines has been of particular interest (e.g., Hawkins et al., 2016; De Haan et al., 2011, 2017). A significant portion of heterocyclic NOCs may also be derived from the heterogeneous aging of secondary organic aerosol (SOA) with ammonia (NH3)/ (Liu et al., 2015; Laskin et al., 2015). Huang et al. (2017) proposed that even trace levels of NH3 may be sufficient to form heterocyclic NOCs via this pathway. However, these pathways have not been confirmed with ambient data, and the relative contribution of heterocyclic NOCs is still uncertain, although they are likely to be minor (at a level of several nanograms per cubic meter, ng m−3) in abundance (Teich et al., 2016).
The secondary formation of NOCs is especially prevalent in environments experiencing high anthropogenic emissions (Yu et al., 2017; Ho et al., 2015), although further studies are required to establish the formation mechanisms comprehensively. A major obstacle is that organic and inorganic matrix effects have a profound impact on the chemistry of organic compounds in bulk aqueous particles and particles undergoing drying (El-Sayed et al., 2015; Lee et al., 2013). While real-time characterization studies remain a challenge due to the extremely complex chemical nature of NOCs, establishing this data along with the covariation of NOCs with other chemical components would help to identify the sources and evolution of NOCs. Using single-particle aerosol time-of-flight mass spectrometry, Wang et al. (2010) observed that the widespread occurrence of NOCs closely correlated with particle acidity in the atmosphere in Shanghai (China). In addition, real-time aerosol mass spectrometry measurements of the atmosphere in New York (US) indicated a definite link between the age of organic species and the N∕C ratio (Sun et al., 2011). Further in-depth studies are required to identify the role of formation conditions, e.g., relative humidity (RH) and pH, for secondary NOCs (Nguyen et al., 2012; Sedehi et al., 2013; Ortiz-Montalvo et al., 2014). In the present study, the mixing state of individual particles was investigated, involving NOCs, oxidized organics, and , based on online seasonal observations using a single-particle aerosol mass spectrometer (SPAMS). Our findings show that the formation of NOCs is significantly linked to oxidized organics and ; this has important environmental implications regarding the assessment of the impact and fate of these compounds.
2.1 Field measurements
Sampling was carried out at the Guangzhou Institute of Geochemistry, a representative urban site in Guangzhou (China), a megacity in the Pearl River Delta (PRD) region. The size and chemical composition of individual particles were obtained by the SPAMS (Hexin Analytical Instrument Co., Ltd., China) in real-time (Li et al., 2011). The sampling inlet for aerosol characterization was situated 40 m above ground level. A brief description of the performance of the SPAMS and other instruments can be found in the Supplement. The sampling periods cover four seasons, including summer (13 June to 16 July 2013), fall (26 September to 19 October 2013), winter (15 to 25 December 2013), and spring (21 February to 11 April 2014). The total measured particle numbers and mean values for meteorological data and gaseous pollutants are outlined for each season in Table S1 in the Supplement and have been described in a previous publication (Zhang et al., 2019).
2.2 SPAMS data analysis
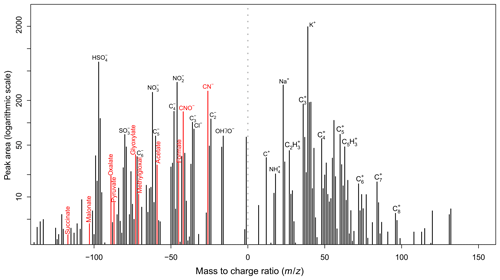
Fragments of NOCs were identified according to the detection of ion peaks at [CN]− or [CNO]−, generally due to the presence of C–N bonds (Silva and Prather, 2000; Zawadowicz et al., 2017; Pagels et al., 2013). Laboratory produced C–N bond compounds from bulk solution-phase reactions between the representative oxidized organics (i.e., methylglyoxal) and ammonium sulfate were used to confirm the generation of ion peaks at [CN]− and/or [CNO]− using SPAMS (Fig. S1 in the Supplement). Thus, the NOCs herein may refer to complex nitrated organics such as organic nitrates, nitroaromatics, nitrogen heterocycles, and polyphenols. Unfortunately, how well ions represented NOCs could not be quantified, although they were the most commonly reported NOC peaks by single-particle mass spectrometry (Silva and Prather, 2000; Zawadowicz et al., 2017; Pagels et al., 2013). In the present study, ions are among the major peaks detected by the SPAMS (Fig. 1). A rough estimate from the peak area ratio of ions and the most likely NOCs fragments (i.e., various amines and an entire series of nitrogen-containing cluster ions CnN−, n=1, 2, 3, …) (Silva and Prather, 2000) shows that ions may represent more than 90 % of these NOCs peaks. The number fractions (Nfs) of particles that contained NOCs ranged from 56 % to 59 % across all four seasons (Table S1). The number of detected NOC-containing particles as a function of their vacuum aerodynamic diameter (dva) is shown in Fig. S2. Most of the detected NOC-containing particles had a dva of between 300 and 1200 nm.
A representative mass spectrum for NOC-containing particles is shown in Fig. 1. Dominant peaks in the mass spectrum were m∕z 39 [K]+, m∕z 23 [Na]+, nitrate ( [NO3]− or [NO2]−), sulfate ( [HSO4]−), organics (m∕z 27 [C2H3]+, m∕z 63 [C5H3]+, [CNO]−, [CN]−), (m∕z 18 [NH4]+), and carbon ion clusters (, n=1, 2, 3, …). NOC-containing particles were internally mixed with various oxidized organics, represented as formate at [HCO2]−, acetate at [CH3CO2]−, methylglyoxal at [C3H3O2]−, glyoxylate at [C2HO3]−, pyruvate at [C3H3O3]−, malonate at [C3H3O4]−, and succinate at [C4H5O4]− (Zhang et al., 2017; Zauscher et al., 2013; Lee et al., 2003). These oxidized organics showed pronounced diurnal trends with an afternoon maximum and were highly correlated (r=0.72–0.94, p < 0.01) with each other. Therefore, they were primarily attributed to secondary oxidized organics from the photochemical oxidation of various volatile organic compounds (VOCs) (Paulot et al., 2011; Zhao et al., 2012; Ho et al., 2011), and the details can be found in our previous publication (Zhang et al., 2019). More information on the seasonal variation range of the Nfs of oxidized organics, , and NOCs is presented in Fig. S3.
Hourly mean Nfs and relative peak areas were applied herein to indicate the variations of aerosol compositions in individual particles. Even though advances have been made in the quantification of specific chemical species for individual particles based on their respective peak area information, it is still quite a challenge for SPAMS to provide quantitative information on aerosol components, mainly due to matrix effects, incomplete ionization, and so forth (Qin et al., 2006; Jeong et al., 2011; Healy et al., 2013; Zhou et al., 2016). Despite this, the variation of the relative peak area should be a good indicator for the investigation of atmospheric processing of various species in individual particles (Wang et al., 2010; Zauscher et al., 2013; Sullivan and Prather, 2007; Zhang et al., 2014).
3.1 Evidence for the formation of NOCs from oxidized organics and ammonium ()
Figure 2 shows the seasonal variations in Nfs of the oxidized organics and , which were internally mixed with NOCs. On average, more than 90 % of the oxidized organics and 65 % of were found to be internally mixed with NOCs, except in spring (Fig. S4). As the Nfs of NOCs relative to all of the measured particles was ∼60 %, it could be concluded that NOCs were enhanced with the presence of oxidized organics and , with the enhancement associated with oxidized organics being the most pronounced.
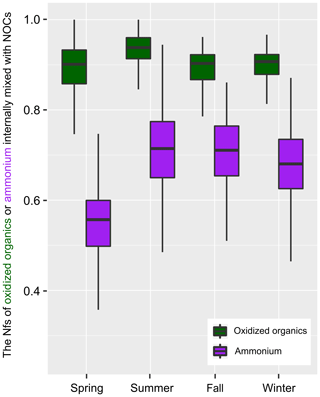
Figure 2The variation in hourly mean Nfs of the oxidized organics and ammonium () that internally mixed with NOCs. The boxes in the box and whisker plot show lower, median, and upper lines, denoting the 25th, 50th, and 75th percentiles, respectively; the whiskers denote the 10th and 90th percentiles, respectively.
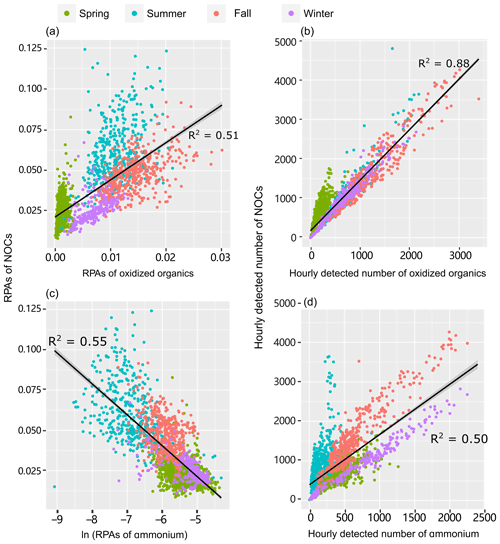
Figure 3Correlation analysis of (a, c) the RPAs and (b, d) the number of detected NOCs with oxidized organics and ammonium () in different seasons. Significant (p < 0.01) correlations were obtained for both the total observed data and the seasonally separated data. A RPA is defined as the fractional peak area of each m∕z relative to the sum of peak areas in the mass spectrum and is applied to represent the relative amount of a species on a particle (Jeong et al., 2011; Healy et al., 2013).
A strong correlation between both the Nfs and relative peak areas (RPAs) of NOCs and oxidized organics further demonstrates their close associations, as shown in Fig. 3. Compared with the oxidized organics, the Nfs of ammonium-containing particles internally mixed with NOCs varied within a broader range (∼40 %–90 %). However, there is still a mixing enhancement of NOCs with . A positive correlation (R2=0.50, p < 0.01) is observed between the hourly detected number of NOCs and . It is worth noting that a negative correlation (R2=0.55, p < 0.01) is obtained between the hourly average RPAs of NOCs and (Fig. 3).
Based on both the enhancement of NOCs and the high correlations with oxidized organics and , it is hypothesized that interactions between oxidized organics and contributed to the observed NOCs. The formation of NOCs from and carbonyls has been confirmed in several laboratory studies (Sareen et al., 2010; Shapiro et al., 2009; Noziere et al., 2009; Kampf et al., 2016; Galloway et al., 2009). Secondary organic aerosols (SOA) produced from a large group of biogenic and anthropogenic VOCs can be further aged by to generate NOCs (Nguyen et al., 2012; Bones et al., 2010; Updyke et al., 2012; Liu et al., 2015; Huang et al., 2017). In a chamber study, the formation of NOCs has been shown to be enhanced in an NH3-rich environment (Chu et al., 2016). While such chemical mechanisms might be complicated, the initial steps generally involve reactions forming imines and amines, which can further react with carbonyl SOA compounds to form more complex products (e.g., oligomers/BrC) (Laskin et al., 2015).
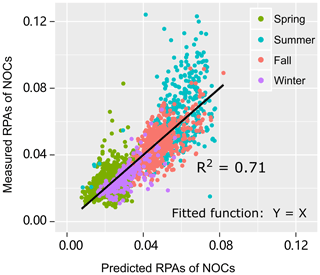
To verify this hypothesis, a multiple linear regression analysis is performed to test how well the RPAs of NOCs could be predicted by oxidized organics and . As expected, there is a close association (R2=0.71, p < 0.01) between the predicted RPAs and the observed values of NOCs (Fig. 4), which supports this hypothesis. A noticeable improvement in the R2 value implies that a model that uses both oxidized organics and to predict RPAs of NOCs is substantially better than a model that only uses a single predictor (either oxidized organics or in Fig. 3). The result indicates that interactions involving oxidized organics and could explain over half of the observed variations in NOCs in the atmosphere in Guangzhou. A fraction of the unaccounted for NOCs could be due to primary emissions and other formation pathways. This hypothesis could also be supported by the similar pattern of diurnal variation observed for NOCs and oxidized organics (Fig. S5), although there is a slight lag for the NOCs. This diurnal pattern is similar to those observed in Beijing and Uintah (Yuan et al., 2016; Zhang et al., 2015). Notably, such a diurnal pattern of secondary NOCs is adequately modeled when the production of NOCs via carbonyls and is included (Woo et al., 2013). In addition to possible photo-bleaching (Zhao et al., 2015), the lower contribution of NOCs during the daytime may be partly explained by the lower RH, as discussed in Sect. 3.2.
Interestingly, the relationship between NOCs and is distinctly different from the relationship between NOCs and oxidized organics (Fig. 3). This implies that the controlling factors regarding the formation of NOCs from are different from oxidized organics. On the one hand, the positive correlation between the detected numbers reflects that the formation of NOCs does require the participation of , which is consistent with the enhancement of NOCs in ammonium-containing particles (Fig. 2) discussed above. On the other hand, the negative correlation between the RPAs signifies that the formation of NOCs is most probably influenced by the relative amount of in individual particles. Such influence could also be supported by our data from both filter samples and individual particle analysis. There is a negative correlation between concentrations of water soluble organic nitrogen (WSON) and for the filter samples (Fig. S6). It can also be seen from Fig. S7 that lower RPAs of correspond to higher Nfs of that internally mixed with NOCs. This inverse correlation could also serve as evidence to explain the influence of the relative amount of on the formation of NOCs.
An influence of the relative amount of on the formation of NOCs is also theoretically possible, as the formation of NOCs may be affected by particle acidity (Miyazaki et al., 2014; Nguyen et al., 2012), which is substantially affected by the abundance of . Higher relative acidity was consistently observed for the internally mixed and NOC particles compared with ammonium-containing particles without NOCs (Fig. S6) and, thus, may influence the formation of NOCs (Fig. S7). Particle acidity could also play a significant role in the gas-to-particle partitioning of aldehydes (Herrmann et al., 2015; Liggio et al., 2005; Gen et al., 2018; De Haan et al., 2018; Kroll et al., 2005), which are precursors for the formation of oxidized organics. However, the higher relative acidity might also be a result of NOC formation. A model simulation shows that after including the chemistry of SOA aging with NH3, an increase in aerosol acidity would be expected due to the reduction in (Zhu et al., 2018). It is also noted that the particle acidity is roughly estimated by the relative abundance of , nitrate, and sulfate in individual particles (Denkenberger et al., 2007); thus, it may not be representative of actual aerosol acidity or pH (Guo et al., 2015; Hennigan et al., 2015; Murphy et al., 2017). In addition, NH3 in the gas phase is also efficient at producing NOCs (Nguyen et al., 2012), which may play an intricate role in the distribution of and NOCs in the particulate phase. The formation of and NOCs would compete for NH3, which may also potentially result in a negative correlation between the RPAs of NOCs and . Unfortunately, such a role remains unclear, as the variations of NH3 were not available in the present study.
3.2 Factors contributing to the NOCs resolved by positive matrix factorization (PMF) analysis
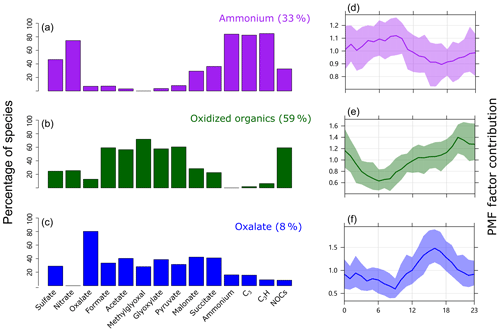
Figure 5 presents the PMF factor profiles obtained from the PMF model analysis (detailed information is provided in the Supplement) (Norris et al., 2009) and their diurnal variations. Around 75 % of NOCs could be well explained by two factors, with 33 % of the PMF-resolved NOCs mainly associated with and carbonaceous ion peaks ( factor), while 59 % were mainly associated with oxidized organics (oxidized organics factor). The fraction of NOCs explained by the and oxidized organic factors is consistent with the linear regression analysis. Furthermore, PMF analysis provided information on the factor contributions and diurnal variations, which may help explain the seasonal variations and processes of NOCs. The ammonium factor showed a diurnal variation pattern that peaked during the early morning, which is consistent with the diurnal variation in RH (Zhang et al., 2019). This factor contributed to ∼80 % (Fig. S8) of the PMF-resolved NOCs during spring (with the highest RH) (Table S1), whereas the oxidized organics factor dominated (> 80 %) in summer and fall. In winter, these two factors had similar contributions (∼40 %). Variation of the ammonium factor may reflect a potential role of aqueous pathways in the formation of NOCs, particularly during spring. In contrast, the oxidized organics factor showed a pattern of diurnal variation, increasing from morning hours and peaking overnight, that may correspond to the photochemical production of oxidized organics and followed interactions with condensed . This pathway may explain the slightly late peak in NOCs compared with oxidized organics, as condensation is favorable overnight (Hu et al., 2008). While there were similarities in the fractions of oxidized organics in the oxalate factor and the oxidized organics factor, they only contributed to 8 % of the PMF-resolved NOCs in the oxalate factor, which contained ∼80 % of the PMF-resolved oxalate. As previously discussed, these oxidized organics are also precursors for the formation of oxalate (Zhang et al., 2019). Therefore, the PMF results suggest that there are two competitive pathways for the evolution of these oxidized organics. Some oxidized organics formed from photochemical activities were further oxidized to oxalate, resulting in a diurnal pattern of variation with concentration peaks during the afternoon (Fig. 5), whereas others interacted with to form NOCs, peaking during the nighttime. However, the controlling factors for these pathways could not be determined in the present study. The unexplained NOCs (∼25 %) might be linked to primary emissions, such as biomass burning (Desyaterik et al., 2013). This could be partly supported by the presence of potassium and various carbon ion clusters (, n=1, 2, 3, …) in the mass spectrum of NOC-containing particles (Fig. 1).
3.3 Seasonal variations in the observed NOCs
There is an evident seasonal variation in NOCs, with higher relative contributions during summer and fall (Figs. 3, 4), mainly due to the variations in oxidized organics and . In this region, a more considerable contribution from secondary oxidized organics is typically observed during summer and fall (Zhou et al., 2014; Yuan et al., 2018). The seasonal maximum NH3 concentrations have also been reported during the warmer seasons, corresponding to the peak emissions from agricultural activities and high temperatures, whereas the low NH3 concentrations observed in colder seasons may be attributed to gas-to-particle conversion (Pan et al., 2018; Zheng et al., 2012). This seasonal variation in NOCs is also obtained in a model simulation, showing that the conversion of NH3 into NOCs would result in a significantly higher reduction of gas-phase NH3 during summer (67 %) than in winter (31 %), due to the higher NH3 and SOA concentrations present in summer (Zhu et al., 2018). More primary NOCs may also be present during summer and fall in the present study, due to the additional biomass burning activities in these seasons (Chen et al., 2018; Zhang et al., 2013).
The seasonal variations in NOCs can be adequately explained by the variations in the concentrations of oxidized organics and (Fig. 4), although the hourly variations during each season are not well explained, as indicated by the lower R2 values (Table S2). The correlation coefficients (R2) range from 0.24 to 0.57 for inter-seasonal variations. During spring, NOCs exhibits a limited dependence on oxidized organics (Fig. 3a, b), while during summer, the hourly detected number of NOCs shows a limited dependence on (Fig. 3d). These seasonal dependences of NOCs are consistent with the PMF results, showing that the ammonium factor explained ∼80 % of the predicted NOCs during spring, whereas the oxidized organics factor dominantly contributed to the predicted NOCs during warmer seasons (Fig. S8). A detailed discussion of this issue is provided in the Supplement.
3.4 Influence of RH and NOx
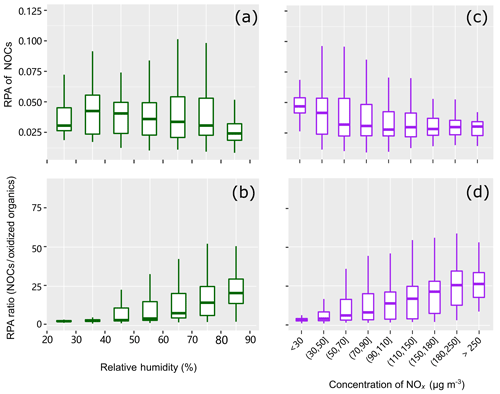
The influence of RH on RPAs of NOCs and peak ratios of NOCs/oxidized organics are shown in Fig. 6. While NOCs do not show a clear dependence on RH, the ratio of NOCs to the oxidized organics shows an apparent increase towards higher RH. This finding is consistent with the observations reported by Xu et al. (2017), in which the N∕C ratio significantly increases as a function of RH in the atmosphere of Beijing. Moreover, the diurnal variations of NOCs with peaks values around 20:00 LT (local time) are also similar to those reported by Xu et al. (2017). The peak ratios of NOCs/oxidized organics are more obviously enhanced when the RH is higher than 40 %. These findings imply that aqueous-phase processing likely plays a substantial role in the formation of NOCs. Significant changes in RH, such as during the evaporation of water droplets, have been reported to facilitate the formation of NOCs via and SOA (Nguyen et al., 2012). In addition, an increase in RH would improve the uptake of NH3 and the formation of , which would also contribute to the enhancement of NOCs. However, the relatively weak correlation (R2=0.27, p < 0.01) between the peak ratios and the RH reflects the complex influence of the RH on the formation of NOCs (Xu et al., 2017; Woo et al., 2013).
One may expect that NOCs are formed through the interactions between NOx and oxidized organics in the gas phase, followed by condensation (Fry et al., 2014; Ziemann and Atkinson, 2012; Seinfeld and Pandis, 2006). Similar to the behavior observed for RH, NOCs do not show a clear dependence on NOx (Fig. 6c, R2=0.02–0.13); however, the ratio of NOCs to the oxidized organics shows a clear increasing trend towards higher NOx (Fig. 6d, R2=0.18, p < 0.01). This indicates that NOx may play a certain role in the conversion of oxidized organics to NOCs, although this cannot be quantified. It is also noted that low correlation coefficients between NOx and NOCs might not indicate a limited contribution of NOx to the formation of NOCs. NOx affects the formation of NOCs in various ways (e.g., peroxy radical chemistry in VOC oxidation mechanisms and the formation of nitrate radicals) (Xu et al., 2015; Zhang et al., 2018) and, thus, may not linearly contribute to the formation of NOCs.
3.5 Atmospheric implications and limitations
In this study, we showed that secondary NOCs were significantly contributed by the heterogeneous aging of oxidized organics with in an urban megacity area, providing valuable insight into SOA aging mechanisms. In particular, the effects of on SOA or BrC formation remain relatively poorly understood. In the PRD region, it has been shown that oxygenated organic aerosols account for more than 40 % of the total organic mass (He et al., 2011), with high concentrations of available gaseous carbonyls (Li et al., 2014). Therefore, it is expected that over half of all water-soluble NOCs in this region might link to secondary processing (Yu et al., 2017). Furthermore, secondary sources have been found to contribute significantly to NOC-related BrC in Nanjing, China (Chen et al., 2018). The results presented here also suggest that the production of NOCs might be adequately estimated by their correlation with secondary oxidized organics and . The effectiveness of correlation-based estimations needs to be examined in other regions before being generally applied in different environments. However, this approach may provide valuable insights into the investigation of NOCs using atmospheric observations. In contrast, it has previously been reported that a positive correlation exists between WSON and (Li et al., 2012), indicating similar anthropogenic sources. This divergence could be mainly attributed to the varying contributions of primary sources and secondary processes to the observed NOCs. Possible future reductions in anthropogenic emissions of NH3 may reduce particulate NOCs. Understanding the complex interplay between inorganic and organic nitrogen is an essential part of assessing global nitrogen cycling.
Moise et al. (2015) proposed that with high concentrations of reduced nitrogen compounds, high photochemical activity, and frequent changes in humidity, BrC formed via and SOA may become a dominant contributor to aerosol absorption, specifically in agricultural and forested areas. However, this study suggests that even in typical urban areas, BrC formation via and SOA should not be neglected. In particular, SOA was found to account for 44 %–71 % of the organic mass in megacities across China (Huang et al., 2014), with NH3 concentrations in urban areas comparable with those from agricultural sites and 2- or 3-fold those of forested areas in China (Pan et al., 2018). Additionally, the acidic nature of particles in these regions would also be favorable for the formation of NOCs (Guo et al., 2017; Jia et al., 2018). Considering the formation of NOCs from the uptake of NH3 onto SOA particles, Zhu et al. (2018) suggested that this mechanism could have a significant impact on the atmospheric concentrations of and .
This study investigated the processes contributing to the seasonal formation of NOCs, involving and oxidized organics in urban Guangzhou, using single-particle mass spectrometry. This is the first study to provide direct field observation results to confirm that the variations in NOCs correlate well and are strongly enhanced by internal mixing with secondary oxidized organics. These findings highlight the possible formation pathway of NOCs via the aging of secondary oxidized organics by in ambient urban environments. A clear pattern of seasonal variation in NOCs was observed, with higher relative contributions in summer and fall compared with winter and spring. This seasonal variation was well predicted by a multiple linear regression model analysis, using the relative abundance of oxidized organics and as model inputs. More than 50 % of NOCs could be explained by the interaction between oxidized organics and . The production of NOCs via such processes is facilitated by increased humidity and NOx. These results extend our understanding of the mixing state and the atmospheric processing of particulate NOCs as well as having substantial implications for the accuracy of models predicting the formation, fate, and impacts of NOCs in the atmosphere.
The dataset related to this article is available online at https://doi.org/10.5281/zenodo.3633443 (Zhang, 2020).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-20-1469-2020-supplement.
GHZ and XHB designed the research (with input from WS, LL, ZYW, DHC, MJT, XMW, and GYS), analyzed the data, and wrote the paper. XFL, YZF, and QHL conducted air sampling work and laboratory experiments under the guidance of GHZ, XHB, and XMW. All authors contributed to the refinement of the paper.
The authors declare that they have no conflict of interest.
This research has been supported by the National Natural Science Foundation of China (grant nos. 41775124 and 41877307), the National Key Research and Development Program of China (grant no. 2017YFC0210104), the Guangdong Foundation for Program of Science and Technology Research (grant no. 2017B030314057), and the Science and Technology Project of Guangzhou, China (grant no. 201803030032). This is contribution no. IS-2810 from CASGIG.
This paper was edited by Sergey A. Nizkorodov and reviewed by two anonymous referees.
Altieri, K. E., Turpin, B. J., and Seitzinger, S. P.: Composition of Dissolved Organic Nitrogen in Continental Precipitation Investigated by Ultra-High Resolution FT-ICR Mass Spectrometry, Environ. Sci. Technol., 43, 6950–6955, https://doi.org/10.1021/es9007849, 2009.
Andreae, M. O. and Gelencsér, A.: Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols, Atmos. Chem. Phys., 6, 3131–3148, https://doi.org/10.5194/acp-6-3131-2006, 2006.
Bones, D. L., Henricksen, D. K., Mang, S. A., Gonsior, M., Bateman, A. P., Nguyen, T. B., Cooper, W. J., and Nizkorodov, S. A.: Appearance of strong absorbers and fluorophores in limonene-O3 secondary organic aerosol due to -mediated chemical aging over long time scales, J. Geophys. Res.-Atmos., 115, D05203, https://doi.org/10.1029/2009jd012864, 2010.
Cape, J. N., Cornell, S. E., Jickells, T. D., and Nemitz, E.: Organic nitrogen in the atmosphere – Where does it come from? A review of sources and methods, Atmos. Res., 102, 30–48, https://doi.org/10.1016/j.atmosres.2011.07.009, 2011.
Chen, Y. F., Ge, X. L., Chen, H., Xie, X. C., Chen, Y. T., Wang, J. F., Ye, Z. L., Bao, M. Y., Zhang, Y. L., and Chen, M. D.: Seasonal light absorption properties of water-soluble brown carbon in atmospheric fine particles in Nanjing, China, Atmos. Environ., 187, 230–240, https://doi.org/10.1016/j.atmosenv.2018.06.002, 2018.
Chu, B., Zhang, X., Liu, Y., He, H., Sun, Y., Jiang, J., Li, J., and Hao, J.: Synergetic formation of secondary inorganic and organic aerosol: effect of SO2 and NH3 on particle formation and growth, Atmos. Chem. Phys., 16, 14219–14230, https://doi.org/10.5194/acp-16-14219-2016, 2016.
De Gouw, J. and Jimenez, J. L.: Organic Aerosols in the Earth's Atmosphere, Environ. Sci. Technol., 43, 7614–7618, https://doi.org/10.1021/Es9006004, 2009.
De Haan, D. O., Hawkins, L. N., Kononenko, J. A., Turley, J. J., Corrigan, A. L., Tolbert, M. A., and Jimenez, J. L.: Formation of Nitrogen-Containing Oligomers by Methylglyoxal and Amines in Simulated Evaporating Cloud Droplets, Environ. Sci. Technol., 45, 984–991, https://doi.org/10.1021/es102933x, 2011.
De Haan, D. O., Hawkins, L. N., Welsh, H. G., Pednekar, R., Casar, J. R., Pennington, E. A., de Loera, A., Jimenez, N. G., Symons, M. A., Zauscher, M., Pajunoja, A., Caponi, L., Cazaunau, M., Formenti, P., Gratien, A., Pangui, E., and Doussin, J.-F.: Brown Carbon Production in Ammonium- or Amine-Containing Aerosol Particles by Reactive Uptake of Methylglyoxal and Photolytic Cloud Cycling, Environ. Sci. Technol., 51, 7458–7466, https://doi.org/10.1021/acs.est.7b00159, 2017.
De Haan, D. O., Jimenez, N. G., de Loera, A., Cazaunau, M., Gratien, A., Pangui, E., and Doussin, J.-F.: Methylglyoxal Uptake Coefficients on Aqueous Aerosol Surfaces, J. Phys. Chem. A, 122, 4854–4860, https://doi.org/10.1021/acs.jpca.8b00533, 2018.
Denkenberger, K. A., Moffet, R. C., Holecek, J. C., Rebotier, T. P., and Prather, K. A.: Real-time, single-particle measurements of oligomers in aged ambient aerosol particles, Environ. Sci. Technol., 41, 5439–5446, https://doi.org/10.1021/es070329l, 2007.
Desyaterik, Y., Sun, Y., Shen, X., Lee, T., Wang, X., Wang, T., and Collett Jr., J. L.: Speciation of “brown” carbon in cloud water impacted by agricultural biomass burning in eastern China, J. Geophys. Res.-Atmos., 118, 7389–7399, https://doi.org/10.1002/jgrd.50561, 2013.
El-Sayed, M. M. H., Wang, Y. Q., and Hennigan, C. J.: Direct atmospheric evidence for the irreversible formation of aqueous secondary organic aerosol, Geophys. Res. Lett., 42, 5577–5586, https://doi.org/10.1002/2015gl064556, 2015.
Feng, Y., Ramanathan, V., and Kotamarthi, V. R.: Brown carbon: a significant atmospheric absorber of solar radiation?, Atmos. Chem. Phys., 13, 8607–8621, https://doi.org/10.5194/acp-13-8607-2013, 2013.
Fry, J. L., Draper, D. C., Barsanti, K. C., Smith, J. N., Ortega, J., Winkle, P. M., Lawler, M. J., Brown, S. S., Edwards, P. M., Cohen, R. C., and Lee, L.: Secondary Organic Aerosol Formation and Organic Nitrate Yield from NO3 Oxidation of Biogenic Hydrocarbons, Environ. Sci. Technol., 48, 11944–11953, https://doi.org/10.1021/es502204x, 2014.
Galloway, M. M., Chhabra, P. S., Chan, A. W. H., Surratt, J. D., Flagan, R. C., Seinfeld, J. H., and Keutsch, F. N.: Glyoxal uptake on ammonium sulphate seed aerosol: reaction products and reversibility of uptake under dark and irradiated conditions, Atmos. Chem. Phys., 9, 3331–3345, https://doi.org/10.5194/acp-9-3331-2009, 2009.
Gen, M., Huang, D. D., and Chan, C. K.: Reactive Uptake of Glyoxal by Ammonium-Containing Salt Particles as a Function of Relative Humidity, Environ. Sci. Technol., 52, 6903–6911, https://doi.org/10.1021/acs.est.8b00606, 2018.
Guo, H., Xu, L., Bougiatioti, A., Cerully, K. M., Capps, S. L., Hite Jr., J. R., Carlton, A. G., Lee, S.-H., Bergin, M. H., Ng, N. L., Nenes, A., and Weber, R. J.: Fine-particle water and pH in the southeastern United States, Atmos. Chem. Phys., 15, 5211–5228, https://doi.org/10.5194/acp-15-5211-2015, 2015.
Guo, H., Weber, R. J., and Nenes, A.: High levels of ammonia do not raise fine particle pH sufficiently to yield nitrogen oxide-dominated sulfate production, Sci. Rep., 7, 12109, https://doi.org/10.1038/s41598-017-11704-0, 2017.
Hawkins, L. N., Lemire, A. N., Galloway, M. M., Corrigan, A. L., Turley, J. J., Espelien, B. M., and De Haan, D. O.: Maillard Chemistry in Clouds and Aqueous Aerosol As a Source of Atmospheric Humic-Like Substances, Environ. Sci. Technol., 50, 7443–7452, https://doi.org/10.1021/acs.est.6b00909, 2016.
He, L. Y., Huang, X. F., Xue, L., Hu, M., Lin, Y., Zheng, J., Zhang, R. Y., and Zhang, Y. H.: Submicron aerosol analysis and organic source apportionment in an urban atmosphere in Pearl River Delta of China using high-resolution aerosol mass spectrometry, J. Geophys. Res.-Atmos., 116, 1–15, https://doi.org/10.1029/2010jd014566, 2011.
Healy, R. M., Sciare, J., Poulain, L., Crippa, M., Wiedensohler, A., Prévôt, A. S. H., Baltensperger, U., Sarda-Estève, R., McGuire, M. L., Jeong, C.-H., McGillicuddy, E., O'Connor, I. P., Sodeau, J. R., Evans, G. J., and Wenger, J. C.: Quantitative determination of carbonaceous particle mixing state in Paris using single-particle mass spectrometer and aerosol mass spectrometer measurements, Atmos. Chem. Phys., 13, 9479–9496, https://doi.org/10.5194/acp-13-9479-2013, 2013.
Hennigan, C. J., Izumi, J., Sullivan, A. P., Weber, R. J., and Nenes, A.: A critical evaluation of proxy methods used to estimate the acidity of atmospheric particles, Atmos. Chem. Phys., 15, 2775–2790, https://doi.org/10.5194/acp-15-2775-2015, 2015.
Herrmann, H., Schaefer, T., Tilgner, A., Styler, S. A., Weller, C., Teich, M., and Otto, T.: Tropospheric Aqueous-Phase Chemistry: Kinetics, Mechanisms, and Its Coupling to a Changing Gas Phase, Chem. Rev., 115, 4259–4334, https://doi.org/10.1021/cr500447k, 2015.
Ho, K. F., Ho, S. S. H., Lee, S. C., Kawamura, K., Zou, S. C., Cao, J. J., and Xu, H. M.: Summer and winter variations of dicarboxylic acids, fatty acids and benzoic acid in PM2.5 in Pearl Delta River Region, China, Atmos. Chem. Phys., 11, 2197–2208, https://doi.org/10.5194/acp-11-2197-2011, 2011.
Ho, K. F., Ho, S. S. H., Huang, R. J., Liu, S. X., Cao, J. J., Zhang, T., Chuang, H. C., Chan, C. S., Hu, D., and Tian, L. W.: Characteristics of water-soluble organic nitrogen in fine particulate matter in the continental area of China, Atmos. Environ., 106, 252–261, https://doi.org/10.1016/j.atmosenv.2015.02.010, 2015.
Hu, M., Wu, Z., Slanina, J., Lin, P., Liu, S., and Zeng, L.: Acidic gases, ammonia and water-soluble ions in PM2.5 at a coastal site in the Pearl River Delta, China, Atmos. Environ., 42, 6310–6320, 2008.
Huang, M., Xu, J., Cai, S., Liu, X., Zhao, W., Hu, C., Gu, X., Fang, L., and Zhang, W.: Characterization of brown carbon constituents of benzene secondary organic aerosol aged with ammonia, J. Atmos. Chem., 75, 205–218, https://doi.org/10.1007/s10874-017-9372-x, 2017.
Huang, R. J., Zhang, Y., Bozzetti, C., Ho, K. F., Cao, J. J., Han, Y., Daellenbach, K. R., Slowik, J. G., Platt, S. M., Canonaco, F., Zotter, P., Wolf, R., Pieber, S. M., Bruns, E. A., Crippa, M., Ciarelli, G., Piazzalunga, A., Schwikowski, M., Abbaszade, G., Schnelle-Kreis, J., Zimmermann, R., An, Z., Szidat, S., Baltensperger, U., El Haddad, I., and Prevot, A. S.: High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 514, 218–222, https://doi.org/10.1038/nature13774, 2014.
Jeong, C.-H., McGuire, M. L., Godri, K. J., Slowik, J. G., Rehbein, P. J. G., and Evans, G. J.: Quantification of aerosol chemical composition using continuous single particle measurements, Atmos. Chem. Phys., 11, 7027–7044, https://doi.org/10.5194/acp-11-7027-2011, 2011.
Jia, S. G., Sarkar, S., Zhang, Q., Wang, X. M., Wu, L. L., Chen, W. H., Huang, M. J., Zhou, S. Z., Zhang, J. P., Yuan, L., and Yang, L. M.: Characterization of diurnal variations of PM2.5 acidity using an open thermodynamic system: A case study of Guangzhou, China, Chemosphere, 202, 677–685, https://doi.org/10.1016/j.chemosphere.2018.03.127, 2018.
Kampf, C. J., Filippi, A., Zuth, C., Hoffmann, T., and Opatz, T.: Secondary brown carbon formation via the dicarbonyl imine pathway: nitrogen heterocycle formation and synergistic effects, Phys. Chem. Chem. Phys., 18, 18353–18364, https://doi.org/10.1039/c6cp03029g, 2016.
Kanakidou, M., Seinfeld, J. H., Pandis, S. N., Barnes, I., Dentener, F. J., Facchini, M. C., Van Dingenen, R., Ervens, B., Nenes, A., Nielsen, C. J., Swietlicki, E., Putaud, J. P., Balkanski, Y., Fuzzi, S., Horth, J., Moortgat, G. K., Winterhalter, R., Myhre, C. E. L., Tsigaridis, K., Vignati, E., Stephanou, E. G., and Wilson, J.: Organic aerosol and global climate modelling: a review, Atmos. Chem. Phys., 5, 1053–1123, https://doi.org/10.5194/acp-5-1053-2005, 2005.
Kroll, J. H., Ng, N. L., Murphy, S. M., Varutbangkul, V., Flagan, R. C., and Seinfeld, J. H.: Chamber studies of secondary organic aerosol growth by reactive uptake of simple carbonyl compounds, J. Geophys. Res.-Atmos., 110, D23207, https://doi.org/10.1029/2005JD006004, 2005.
Laskin, A., Smith, J. S., and Laskin, J.: Molecular Characterization of Nitrogen-Containing Organic Compounds in Biomass Burning Aerosols Using High-Resolution Mass Spectrometry, Environ. Sci. Technol., 43, 3764–3771, https://doi.org/10.1021/es803456n, 2009.
Laskin, A., Laskin, J., and Nizkorodov, S. A.: Chemistry of Atmospheric Brown Carbon, Chem. Rev., 115, 4335–4382, https://doi.org/10.1021/cr5006167, 2015.
Lee, A. K. Y., Zhao, R., Li, R., Liggio, J., Li, S. M., and Abbatt, J. P. D.: Formation of Light Absorbing Organo-Nitrogen Species from Evaporation of Droplets Containing Glyoxal and Ammonium Sulfate, Environ. Sci. Technol., 47, 12819–12826, https://doi.org/10.1021/es402687w, 2013.
Lee, S. H., Murphy, D. M., Thomson, D. S., and Middlebrook, A. M.: Nitrate and oxidized organic ions in single particle mass spectra during the 1999 Atlanta Supersite Project, J. Geophys. Res., 108, 8417, https://doi.org/10.1029/2001jd001455, 2003.
Li, J., Fang, Y. T., Yoh, M., Wang, X. M., Wu, Z. Y., Kuang, Y. W., and Wen, D. Z.: Organic nitrogen deposition in precipitation in metropolitan Guangzhou city of southern China, Atmos. Res., 113, 57–67, https://doi.org/10.1016/j.atmosres.2012.04.019, 2012.
Li, L., Huang, Z. X., Dong, J. G., Li, M., Gao, W., Nian, H. Q., Fu, Z., Zhang, G. H., Bi, X. H., Cheng, P., and Zhou, Z.: Real time bipolar time-of-flight mass spectrometer for analyzing single aerosol particles, Int. J. Mass. Spectrom., 303, 118–124, https://doi.org/10.1016/j.ijms.2011.01.017, 2011.
Li, X., Rohrer, F., Brauers, T., Hofzumahaus, A., Lu, K., Shao, M., Zhang, Y. H., and Wahner, A.: Modeling of HCHO and CHOCHO at a semi-rural site in southern China during the PRIDE-PRD2006 campaign, Atmos. Chem. Phys., 14, 12291–12305, https://doi.org/10.5194/acp-14-12291-2014, 2014.
Li, Z., Nizkorodov, S. A., Chen, H., Lu, X., Yang, X., and Chen, J.: Nitrogen-containing secondary organic aerosol formation by acrolein reaction with ammonia/ammonium, Atmos. Chem. Phys., 19, 1343–1356, https://doi.org/10.5194/acp-19-1343-2019, 2019.
Liggio, J., Li, S. M., and Mclaren, R.: Reactive uptake of glyoxal by particulate matter, J. Geophys. Res.-Atmos., 110, D10304, https://doi.org/10.1029/2004jd005113, 2005.
Lin, P., Aiona, P. K., Li, Y., Shiraiwa, M., Laskin, J., Nizkorodov, S. A., and Laskin, A.: Molecular Characterization of Brown Carbon in Biomass Burning Aerosol Particles, Environ. Sci. Technol., 50, 11815–11824, https://doi.org/10.1021/acs.est.6b03024, 2016.
Liu, Y., Liggio, J., Staebler, R., and Li, S.-M.: Reactive uptake of ammonia to secondary organic aerosols: kinetics of organonitrogen formation, Atmos. Chem. Phys., 15, 13569–13584, https://doi.org/10.5194/acp-15-13569-2015, 2015.
Mace, K. A., Kubilay, N., and Duce, R. A.: Organic nitrogen in rain and aerosol in the eastern Mediterranean atmosphere: An association with atmospheric dust, J. Geophys. Res.-Atmos., 108, 4320, https://doi.org/10.1029/2002jd002997, 2003.
Miyazaki, Y., Fu, P. Q., Ono, K., Tachibana, E., and Kawamura, K.: Seasonal cycles of water-soluble organic nitrogen aerosols in a deciduous broadleaf forest in northern Japan, J. Geophys. Res.-Atmos., 119, 1440–1454, https://doi.org/10.1002/2013JD020713, 2014.
Mohr, C., Lopez-Hilfiker, F. D., Zotter, P., Prévôt, A. S. H., Xu, L., Ng, N. L., Herndon, S. C., Williams, L. R., Franklin, J. P., Zahniser, M. S., Worsnop, D. R., Knighton, W. B., Aiken, A. C., Gorkowski, K. J., Dubey, M. K., Allan, J. D., and Thornton, J. A.: Contribution of Nitrated Phenols to Wood Burning Brown Carbon Light Absorption in Detling, United Kingdom during Winter Time, Environ. Sci. Technol., 47, 6316–6324, https://doi.org/10.1021/es400683v, 2013.
Moise, T., Flores, J. M., and Rudich, Y.: Optical Properties of Secondary Organic Aerosols and Their Changes by Chemical Processes, Chem. Rev., 115, 4400–4439, https://doi.org/10.1021/cr5005259, 2015.
Murphy, J. G., Gregoire, P. K., Tevlin, A. G., Wentworth, G. R., Ellis, R. A., Markovic, M. Z., and VandenBoer, T. C.: Observational constraints on particle acidity using measurements and modelling of particles and gases, Faraday Discuss., 200, 379–395, https://doi.org/10.1039/c7fd00086c, 2017.
Neff, J. C., Holland, E. A., Dentener, F. J., McDowell, W. H., and Russell, K. M.: The origin, composition and rates of organic nitrogen deposition: A missing piece of the nitrogen cycle?, Biogeochemistry, 57, 99–136, 2002.
Nguyen, T. B., Lee, P. B., Updyke, K. M., Bones, D. L., Laskin, J., Laskin, A., and Nizkorodov, S. A.: Formation of nitrogen- and sulfur-containing light-absorbing compounds accelerated by evaporation of water from secondary organic aerosols, J. Geophys. Res.-Atmos., 117, D01207, https://doi.org/10.1029/2011jd016944, 2012.
Norris, G., Vedantham, R., Wade, K., Zahn, P., Brown, S., Paatero, P., Eberly, S., and Foley, C.: Guidance document for PMF applications with the Multilinear Engine, edited, Prepared for the U.S. Environmental Protection Agency, Research Triangle Park, NC, 2009.
Noziere, B., Dziedzic, P., and Cordova, A.: Products and Kinetics of the Liquid-Phase Reaction of Glyoxal Catalyzed by Ammonium Ions (), J. Phys. Chem. A, 113, 231–237, https://doi.org/10.1021/jp8078293, 2009.
Ortiz-Montalvo, D. L., Hakkinen, S. A. K., Schwier, A. N., Lim, Y. B., McNeill, V. F., and Turpin, B. J.: Ammonium Addition (and Aerosol pH) Has a Dramatic Impact on the Volatility and Yield of Glyoxal Secondary Organic Aerosol, Environ. Sci. Technol., 48, 255–262, https://doi.org/10.1021/es4035667, 2014.
Pagels, J., Dutcher, D. D., Stolzenburg, M. R., McMurry, P. H., Galli, M. E., and Gross, D. S.: Fine-particle emissions from solid biofuel combustion studied with single-particle mass spectrometry: Identification of markers for organics, soot, and ash components, J. Geophys. Res.-Atmos., 118, 859–870, https://doi.org/10.1029/2012jd018389, 2013.
Pan, Y. P., Tian, S. L., Zhao, Y. H., Zhang, L., Zhu, X. Y., Gao, J., Huang, W., Zhou, Y. B., Song, Y., Zhang, Q., and Wang, Y. S.: Identifying Ammonia Hotspots in China Using a National Observation Network, Environ. Sci. Technol., 52, 3926–3934, https://doi.org/10.1021/acs.est.7b05235, 2018.
Paulot, F., Wunch, D., Crounse, J. D., Toon, G. C., Millet, D. B., DeCarlo, P. F., Vigouroux, C., Deutscher, N. M., González Abad, G., Notholt, J., Warneke, T., Hannigan, J. W., Warneke, C., de Gouw, J. A., Dunlea, E. J., De Mazière, M., Griffith, D. W. T., Bernath, P., Jimenez, J. L., and Wennberg, P. O.: Importance of secondary sources in the atmospheric budgets of formic and acetic acids, Atmos. Chem. Phys., 11, 1989–2013, https://doi.org/10.5194/acp-11-1989-2011, 2011.
Qin, X. Y., Bhave, P. V., and Prather, K. A.: Comparison of two methods for obtaining quantitative mass concentrations from aerosol time-of-flight mass spectrometry measurements, Anal. Chem., 78, 6169–6178, https://doi.org/10.1021/ac060395q, 2006.
Rastogi, N., Zhang, X., Edgerton, E. S., Ingall, E., and Weber, R. J.: Filterable water-soluble organic nitrogen in fine particles over the southeastern USA during summer, Atmos. Environ., 45, 6040–6047, https://doi.org/10.1016/j.atmosenv.2011.07.045, 2011.
Sareen, N., Schwier, A. N., Shapiro, E. L., Mitroo, D., and McNeill, V. F.: Secondary organic material formed by methylglyoxal in aqueous aerosol mimics, Atmos. Chem. Phys., 10, 997–1016, https://doi.org/10.5194/acp-10-997-2010, 2010.
Sedehi, N., Takano, H., Blasic, V. A., Sullivan, K. A., and De Haan, D. O.: Temperature- and pH-dependent aqueous-phase kinetics of the reactions of glyoxal and methylglyoxal with atmospheric amines and ammonium sulfate, Atmos. Environ., 77, 656–663, https://doi.org/10.1016/j.atmosenv.2013.05.070, 2013.
Seinfeld, J. H. and Pandis, S. N.: Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, John Wiley&Sons, Inc., New Jersey, 2006.
Shapiro, E. L., Szprengiel, J., Sareen, N., Jen, C. N., Giordano, M. R., and McNeill, V. F.: Light-absorbing secondary organic material formed by glyoxal in aqueous aerosol mimics, Atmos. Chem. Phys., 9, 2289–2300, https://doi.org/10.5194/acp-9-2289-2009, 2009.
Shi, J., Gao, H., Qi, J., Zhang, J., and Yao, X.: Sources, compositions, and distributions of water-soluble organic nitrogen in aerosols over the China Sea, J. Geophys. Res.-Atmos., 115, D17303, https://doi.org/10.1029/2009jd013238, 2010.
Shrivastava, M., Cappa, C. D., Fan, J. W., Goldstein, A. H., Guenther, A. B., Jimenez, J. L., Kuang, C., Laskin, A., Martin, S. T., Ng, N. L., Petaja, T., Pierce, J. R., Rasch, P. J., Roldin, P., Seinfeld, J. H., Shilling, J., Smith, J. N., Thornton, J. A., Volkamer, R., Wang, J., Worsnop, D. R., Zaveri, R. A., Zelenyuk, A., and Zhang, Q.: Recent advances in understanding secondary organic aerosol: Implications for global climate forcing, Rev. Geophys., 55, 509–559, https://doi.org/10.1002/2016RG000540, 2017.
Silva, P. J. and Prather, K. A.: Interpretation of mass spectra from organic compounds in aerosol time-of-flight mass spectrometry, Anal. Chem., 72, 3553–3562, 2000.
Sullivan, R. C. and Prather, K. A.: Investigations of the diurnal cycle and mixing state of oxalic acid in individual particles in Asian aerosol outflow, Environ. Sci. Technol., 41, 8062–8069, 2007.
Sun, J., Zhi, G., Hitzenberger, R., Chen, Y., Tian, C., Zhang, Y., Feng, Y., Cheng, M., Zhang, Y., Cai, J., Chen, F., Qiu, Y., Jiang, Z., Li, J., Zhang, G., and Mo, Y.: Emission factors and light absorption properties of brown carbon from household coal combustion in China, Atmos. Chem. Phys., 17, 4769–4780, https://doi.org/10.5194/acp-17-4769-2017, 2017.
Sun, Y.-L., Zhang, Q., Schwab, J. J., Demerjian, K. L., Chen, W.-N., Bae, M.-S., Hung, H.-M., Hogrefe, O., Frank, B., Rattigan, O. V., and Lin, Y.-C.: Characterization of the sources and processes of organic and inorganic aerosols in New York city with a high-resolution time-of-flight aerosol mass apectrometer, Atmos. Chem. Phys., 11, 1581–1602, https://doi.org/10.5194/acp-11-1581-2011, 2011.
Teich, M., van Pinxteren, D., Kecorius, S., Wang, Z. B., and Herrmann, H.: First Quantification of Imidazoles in Ambient Aerosol Particles: Potential Photosensitizers, Brown Carbon Constituents, and Hazardous Components, Environ. Sci. Technol., 50, 1166–1173, https://doi.org/10.1021/acs.est.5b05474, 2016.
Updyke, K. M., Nguyen, T. B., and Nizkorodov, S. A.: Formation of brown carbon via reactions of ammonia with secondary organic aerosols from biogenic and anthropogenic precursors, Atmos. Environ., 63, 22–31, https://doi.org/10.1016/j.atmosenv.2012.09.012, 2012.
Wang, X. F., Gao, S., Yang, X., Chen, H., Chen, J. M., Zhuang, G. S., Surratt, J. D., Chan, M. N., and Seinfeld, J. H.: Evidence for High Molecular Weight Nitrogen-Containing Organic Salts in Urban Aerosols, Environ. Sci. Technol., 44, 4441–4446, 2010.
Wang, X. F., Wang, H. L., Jing, H., Wang, W. N., Cui, W. D., Williams, B. J., and Biswas, P.: Formation of Nitrogen-Containing Organic Aerosol during Combustion of High-Sulfur-Content Coal, Energ. Fuel., 31, 14161–14168, https://doi.org/10.1021/acs.energyfuels.7b02273, 2017.
Woo, J. L., Kim, D. D., Schwier, A. N., Li, R. Z., and McNeill, V. F.: Aqueous aerosol SOA formation: impact on aerosol physical properties, Faraday Discuss., 165, 357–367, https://doi.org/10.1039/c3fd00032j, 2013.
Xu, L., Guo, H. Y., Boyd, C. M., Klein, M., Bougiatioti, A., Cerully, K. M., Hite, J. R., Isaacman-VanWertz, G., Kreisberg, N. M., Knote, C., Olson, K., Koss, A., Goldstein, A. H., Hering, S. V., de Gouw, J., Baumann, K., Lee, S. H., Nenes, A., Weber, R. J., and Ng, N. L.: Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States, P. Natl. Acad. Sci. USA, 112, E4509–E4509, https://doi.org/10.1073/pnas.1512279112, 2015.
Xu, W. Q., Sun, Y. L., Wang, Q. Q., Du, W., Zhao, J., Ge, X. L., Han, T. T., Zhang, Y. J., Zhou, W., Li, J., Fu, P. Q., Wang, Z. F., and Worsnop, D. R.: Seasonal Characterization of Organic Nitrogen in Atmospheric Aerosols Using High Resolution Aerosol Mass Spectrometry in Beijing, China, ACS Earth Space Chem., 1, 673–682, https://doi.org/10.1021/acsearthspacechem.7b00106, 2017.
Yan, J., Wang, X., Gong, P., Wang, C., and Cong, Z.: Review of brown carbon aerosols: Recent progress and perspectives, Sci. Total. Environ., 634, 1475–1485, https://doi.org/10.1016/j.scitotenv.2018.04.083, 2018.
Yu, X., Yu, Q. Q., Zhu, M., Tang, M. J., Li, S., Yang, W. Q., Zhang, Y. L., Deng, W., Li, G. H., Yu, Y. G., Huang, Z. H., Song, W., Ding, X., Hu, Q. H., Li, J., Bi, X. H., and Wang, X. M.: Water Soluble Organic Nitrogen (WSON) in Ambient Fine Particles Over a Megacity in South China: Spatiotemporal Variations and Source Apportionment, J. Geophys. Res.-Atmos., 122, 13045–13060, https://doi.org/10.1002/2017JD027327, 2017.
Yuan, B., Liggio, J., Wentzell, J., Li, S.-M., Stark, H., Roberts, J. M., Gilman, J., Lerner, B., Warneke, C., Li, R., Leithead, A., Osthoff, H. D., Wild, R., Brown, S. S., and de Gouw, J. A.: Secondary formation of nitrated phenols: insights from observations during the Uintah Basin Winter Ozone Study (UBWOS) 2014, Atmos. Chem. Phys., 16, 2139–2153, https://doi.org/10.5194/acp-16-2139-2016, 2016.
Yuan, Q., Lai, S., Song, J., Ding, X., Zheng, L., Wang, X., Zhao, Y., Zheng, J., Yue, D., Zhong, L., Niu, X., and Zhang, Y.: Seasonal cycles of secondary organic aerosol tracers in rural Guangzhou, Southern China: The importance of atmospheric oxidants, Environ. Pollut., 240, 884–893, https://doi.org/10.1016/j.envpol.2018.05.009, 2018.
Zauscher, M. D., Wang, Y., Moore, M. J. K., Gaston, C. J., and Prather, K. A.: Air Quality Impact and Physicochemical Aging of Biomass Burning Aerosols during the 2007 San Diego Wildfires, Environ. Sci. Technol., 47, 7633–7643, https://doi.org/10.1021/es4004137, 2013.
Zawadowicz, M. A., Froyd, K. D., Murphy, D. M., and Cziczo, D. J.: Improved identification of primary biological aerosol particles using single-particle mass spectrometry, Atmos. Chem. Phys., 17, 7193–7212, https://doi.org/10.5194/acp-17-7193-2017, 2017.
Zhang, G.: Dataset for ACP publication titled “High secondary formation of nitrogen-containing organics (NOCs) and its possible link to oxidized organics and ammonium” [Data set], Zenodo, https://doi.org/10.5281/zenodo.3633443, 2020.
Zhang, G., Lin, Q., Peng, L., Yang, Y., Fu, Y., Bi, X., Li, M., Chen, D., Chen, J., Cai, Z., Wang, X., Peng, P., Sheng, G., and Zhou, Z.: Insight into the in-cloud formation of oxalate based on in situ measurement by single particle mass spectrometry, Atmos. Chem. Phys., 17, 13891–13901, https://doi.org/10.5194/acp-17-13891-2017, 2017.
Zhang, G., Lin, Q., Peng, L., Yang, Y., Jiang, F., Liu, F., Song, W., Chen, D., Cai, Z., Bi, X., Miller, M., Tang, M., Huang, W., Wang, X., Peng, P., and Sheng, G.: Oxalate Formation Enhanced by Fe-Containing Particles and Environmental Implications, Environ. Sci. Technol., 53, 1269–1277, https://doi.org/10.1021/acs.est.8b05280, 2019.
Zhang, G. H., Bi, X. H., He, J. J., Chen, D. H., Chan, L. Y., Xie, G. W., Wang, X. M., Sheng, G. Y., Fu, J. M., and Zhou, Z.: Variation of secondary coatings associated with elemental carbon by single particle analysis, Atmos. Environ., 92, 162–170, https://doi.org/10.1016/j.atmosenv.2014.04.018, 2014.
Zhang, H. F., Yee, L. D., Lee, B. H., Curtis, M. P., Worton, D. R., Isaacman-VanWertz, G., Offenberg, J. H., Lewandowski, M., Kleindienst, T. E., Beaver, M. R., Holder, A. L., Lonneman, W. A., Docherty, K. S., Jaoui, M., Pye, H. O. T., Hu, W. W., Day, D. A., Campuzano-Jost, P., Jimenez, J. L., Guo, H. Y., Weber, R. J., de Gouw, J., Koss, A. R., Edgerton, E. S., Brune, W., Mohr, C., Lopez-Hilfiker, F. D., Lutz, A., Kreisberg, N. M., Spielman, S. R., Hering, S. V., Wilson, K. R., Thornton, J. A., and Goldstein, A. H.: Monoterpenes are the largest source of summertime organic aerosol in the southeastern United States, P. Natl. Acad. Sci. USA, 115, 2038–2043, https://doi.org/10.1073/pnas.1717513115, 2018.
Zhang, Q., Duan, F., He, K., Ma, Y., Li, H., Kimoto, T., and Zheng, A.: Organic nitrogen in PM2.5 in Beijing, Front. Env. Sci. Eng., 9, 1004–1014, https://doi.org/10.1007/s11783-015-0799-5, 2015.
Zhang, Y. S., Shao, M., Lin, Y., Luan, S. J., Mao, N., Chen, W. T., and Wang, M.: Emission inventory of carbonaceous pollutants from biomass burning in the Pearl River Delta Region, China, Atmos. Environ., 76, 189–199, https://doi.org/10.1016/j.atmosenv.2012.05.055, 2013.
Zhao, R., Lee, A. K. Y., and Abbatt, J. P. D.: Investigation of Aqueous-Phase Photooxidation of Glyoxal and Methylglyoxal by Aerosol Chemical Ionization Mass Spectrometry: Observation of Hydroxyhydroperoxide Formation, J. Phys. Chem. A, 116, 6253–6263, https://doi.org/10.1021/jp211528d, 2012.
Zhao, R., Lee, A. K. Y., Huang, L., Li, X., Yang, F., and Abbatt, J. P. D.: Photochemical processing of aqueous atmospheric brown carbon, Atmos. Chem. Phys., 15, 6087–6100, https://doi.org/10.5194/acp-15-6087-2015, 2015.
Zheng, J. Y., Yin, S. S., Kang, D. W., Che, W. W., and Zhong, L. J.: Development and uncertainty analysis of a high-resolution NH3 emissions inventory and its implications with precipitation over the Pearl River Delta region, China, Atmos. Chem. Phys., 12, 7041–7058, https://doi.org/10.5194/acp-12-7041-2012, 2012.
Zhou, S. Z., Wang, T., Wang, Z., Li, W. J., Xu, Z., Wang, X. F., Yuan, C., Poon, C. N., Louie, P. K. K., Luk, C. W. Y., and Wang, W. X.: Photochemical evolution of organic aerosols observed in urban plumes from Hong Kong and the Pearl River Delta of China, Atmos. Environ., 88, 219–229, https://doi.org/10.1016/j.atmosenv.2014.01.032, 2014.
Zhou, Y., Huang, X. H. H., Griffith, S. M., Li, M., Li, L., Zhou, Z., Wu, C., Meng, J. W., Chan, C. K., Louie, P. K. K., and Yu, J. Z.: A field measurement based scaling approach for quantification of major ions, organic carbon, and elemental carbon using a single particle aerosol mass spectrometer, Atmos. Environ., 143, 300–312, https://doi.org/10.1016/j.atmosenv.2016.08.054, 2016.
Zhu, S., Horne, J. R., Montoya-Aguilera, J., Hinks, M. L., Nizkorodov, S. A., and Dabdub, D.: Modeling reactive ammonia uptake by secondary organic aerosol in CMAQ: application to the continental US, Atmos. Chem. Phys., 18, 3641–3657, https://doi.org/10.5194/acp-18-3641-2018, 2018.
Ziemann, P. J. and Atkinson, R.: Kinetics, products, and mechanisms of secondary organic aerosol formation, Chem. Soc. Rev., 41, 6582–6605, https://doi.org/10.1039/c2cs35122f, 2012.