the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Investigation of the oxidation of methyl vinyl ketone (MVK) by OH radicals in the atmospheric simulation chamber SAPHIR
Sascha Albrecht
Ismail–Hakki Acir
Birger Bohn
Martin Breitenlechner
Hans-Peter Dorn
Georgios I. Gkatzelis
Andreas Hofzumahaus
Frank Holland
Martin Kaminski
Frank N. Keutsch
Anna Novelli
David Reimer
Franz Rohrer
Ralf Tillmann
Luc Vereecken
Robert Wegener
Alexander Zaytsev
Astrid Kiendler-Scharr
Andreas Wahner
The photooxidation of methyl vinyl ketone (MVK) was investigated in the atmospheric simulation chamber SAPHIR for conditions at which organic peroxy radicals (RO2) mainly reacted with NO (“high NO” case) and for conditions at which other reaction channels could compete (“low NO” case). Measurements of trace gas concentrations were compared to calculated concentration time series applying the Master Chemical Mechanism (MCM version 3.3.1). Product yields of methylglyoxal and glycolaldehyde were determined from measurements. For the high NO case, the methylglyoxal yield was (19 ± 3) % and the glycolaldehyde yield was (65 ± 14) %, consistent with recent literature studies. For the low NO case, the methylglyoxal yield reduced to (5 ± 2) % because other RO2 reaction channels that do not form methylglyoxal became important. Consistent with literature data, the glycolaldehyde yield of (37 ± 9) % determined in the experiment was not reduced as much as implemented in the MCM, suggesting additional reaction channels producing glycolaldehyde. At the same time, direct quantification of OH radicals in the experiments shows the need for an enhanced OH radical production at low NO conditions similar to previous studies investigating the oxidation of the parent VOC isoprene and methacrolein, the second major oxidation product of isoprene. For MVK the model–measurement discrepancy was up to a factor of 2. Product yields and OH observations were consistent with assumptions of additional RO2 plus HO2 reaction channels as proposed in literature for the major RO2 species formed from the reaction of MVK with OH. However, this study shows that also HO2 radical concentrations are underestimated by the model, suggesting that additional OH is not directly produced from RO2 radical reactions, but indirectly via increased HO2. Quantum chemical calculations show that HO2 could be produced from a fast 1,4-H shift of the second most important MVK derived RO2 species (reaction rate constant 0.003 s−1). However, additional HO2 from this reaction was not sufficiently large to bring modelled HO2 radical concentrations into agreement with measurements due to the small yield of this RO2 species. An additional reaction channel of the major RO2 species with a reaction rate constant of (0.006 ± 0.004) s−1 would be required that produces concurrently HO2 radicals and glycolaldehyde to achieve model–measurement agreement. A unimolecular reaction similar to the 1,5-H shift reaction that was proposed in literature for RO2 radicals from MVK would not explain product yields for conditions of experiments in this study. A set of H-migration reactions for the main RO2 radicals were investigated by quantum chemical and theoretical kinetic methodologies, but did not reveal a contributing route to HO2 radicals or glycolaldehyde.
- Article
(7518 KB) -
Supplement
(4475 KB) - BibTeX
- EndNote
Isoprene (C5H8) emitted by plants (Guenther et al., 2012) has the highest emission rate among non-methane organic compounds. Isoprene is mainly oxidised by the photochemically generated hydroxyl radical (OH) forming the first-generation organic compounds methacrolein (MACR), methyl vinyl ketone (MVK), formaldehyde (HCHO and isoprene hydroxyperoxides (ISOPOOH) (e.g. Karl et al., 2006). The latter ones are formed without the involvement of nitric oxide (NO) so that ISOPOOH becomes increasingly important with decreasing concentrations of nitrogen oxides (St. Clair et al., 2015) which are mainly released by anthropogenic activities. The formation of MVK and MACR is accompanied by the production of HO2, which can further recycle OH, whereas ISOPOOH formation is a radical termination reaction. However, field studies have shown that also in environments where NO concentrations are less than a few 100 pptv a high OH regeneration rate can be maintained which is not explained by chemical models (Tan et al., 2001; Lelieveld et al., 2008; Hofzumahaus et al., 2009; Whalley et al., 2011). The gap between measured and modelled OH is correlated with the abundance of isoprene (Lu et al., 2012). Since then, it has been recognised that organic peroxy radical (RO2) pathways which do not require NO as reaction partner can also significantly recycle OH (Wennberg et al., 2018). These reactions include
-
hydrogen-shift reactions of RO2 radicals forming OH or HO2,
-
reaction of peroxy radicals with HO2.
These pathways have both been identified in the oxidation chain of isoprene (Peeters et al., 2009, 2014). A 1,6 H-shift of RO2 formed in the reaction of isoprene with OH leads to the formation of hydroxyperoxy aldehydes (HPALD) and HO2. The photolysis of HPALD gives even additional OH radicals. Its relevance for the atmosphere has been shown in laboratory experiments (Crounse et al., 2011; Wolfe et al., 2012) and chamber experiments (Fuchs et al., 2013). In the supplement of the first publication of the LIM (Peeters et al., 2009) the authors also suggested that a 1,5-H-shift reaction could be relevant for MVK and MACR. However, for MACR a 1,4 H-shift reaction for RO2 was found, which efficiently recycles OH (Crounse et al., 2012). Its impact on the radical budget has been shown in chamber experiments (Fuchs et al., 2014). H-shift reactions have also been proposed to be important for RO2 radicals which are formed in the oxidation chain of ISOPOOH (D'Ambro et al., 2017) also potentially enhancing the OH regeneration rate.
The reaction of peroxy radicals with HO2 forms not only hydroxyperoxides, but also OH together with an alkoxy radical. This was shown for the acetylperoxy radical with an OH yield of 50 % (Dillon and Crowley, 2008; Winiberg et al., 2016). In a recent study by Praske et al. (2015), product yields from the reaction of MVK derived peroxy radicals with HO2 were investigated. Similar to the acetylperoxy radical, product yields demonstrated that only one third of the reaction yields hydroxyperoxides and that two additional reaction channels exist, both of which could lead to the reformation of OH.
In this study, the oxidation of MVK by OH was investigated in the atmospheric simulation chamber SAPHIR (Simulation of Atmospheric Photochemistry In a Large Reaction Chamber) at Forschungszentrum Jülich. Experiments were performed under controlled conditions with atmospheric trace gas and radical concentrations. In these experiments, not only organic compounds like in previous studies were measured, but also radical species (OH, HO2, and RO2) allowing for an analysis of the OH budget. In the low NO case, NO mixing ratios were kept below 100 pptv so that different RO2 radical reactions, i.e. reaction with HO2 and unimolecular isomerisation reactions, can compete. Measured time series of radical concentrations are compared to model calculations applying the Master Chemical Mechanism version 3.1.1 (MCM, 2017) and modifications suggested in literature.
2.1 Simulation experiment in SAPHIR
Experiments were performed in the outdoor atmospheric simulation chamber SAPHIR. Details of the chamber can be found in previous publications (e.g. Rohrer et al., 2005). SAPHIR has a cylindrical shape (length 18 m, diameter 5 m, volume 270 m3 and consists of a double-wall FEP (fluorethylene-propylene) film. A small overpressure (45 Pa) prevents ambient air entering the chamber. The replenishment flow that is required to maintain this pressure leads to a dilution of all trace gases by approximately 3 to 5 % per hour. A shutter system shades the chamber before the photooxidation experiment is started. Natural sunlight is used to irradiate the mixture. Small sources of nitrous acid (HONO) and formaldehyde (HCHO) are present in the sunlit chamber (100 to 200 pptv h−1). The photolysis of HONO is typically the primary source for OH radicals and nitrogen oxides.
In total, four experiments were conducted in this study, two of them at low NO (23 June 2016: NO < 70 pptv and 23 May 2017: NO < 40 pptv) and two of them at high NO conditions (20 August 2014: 0.7 to 6 ppbv NO and 17 May 2017: approximately 0.1 to 0.4 ppbv NO). Results from experiments performed at similar conditions gave consistent results. The discussion of results focuses on the two experiments for which the number of trace gas measurements was highest and results for the other experiments are shown in the Supplement.
The experiments started with cleaning the chamber air by flushing out impurities from previous experiments until trace gas concentrations were below the detection limit of the instruments. The chamber air was first humidified by flushing water vapour from boiling water into the dark chamber (relative humidity approximately 70 %). In the low NO experiments, approximately 140 ppbv ozone produced from a silent discharge ozoniser (O3onia) was injected in the dark in order to suppress NO concentrations. In contrast, 6 to 10 ppbv of NO2 or NO were injected from a gas mixture in case of the high NO experiments. In one of the two low NO experiments (23 May 2017), 20 ppbv MVK (Sigma-Aldrich, purity 99 %) in water was injected in the dark chamber from a Liquid Calibration Unit (LCU, Ionicon). MVK (1.5 ppbv) was reinjected after 3.5 h of photooxidation. In the other experiments, MVK was injected several times during the experiment after an initial phase of illumination of the chamber air without additional OH reactants by injecting liquid MVK into a heated inlet line that is flushed by synthetic air. This procedure was similar to that applied in previous studies (e.g. Fuchs et al., 2013, 2014; Kaminski et al., 2017). The photooxidation of MVK was then observed for several hours. No significant particle formation was observed in the experiments so that only gas-phase chemistry needs to be considered in the evaluation.
2.2 Instrumentation
Trace gas concentrations were measured with a comprehensive set of instruments. Nitric oxide (NO) was detected by chemiluminescence (Eco Physics) and nitrogen dioxide (NO2) by the same instrument but with a blue-light converter in the inlet. In one of the experiments (17 May 2017), no NOx measurements were available. A cavity ring-down instrument (Picarro) monitored water vapour and carbon monoxide and a UV photometer (Ansyco) detected ozone.
The total OH reactivity (inverse lifetime of OH) was measured by a pump-probe method (Lou et al., 2010; Fuchs et al., 2017), in which the decay of OH radicals produced by laser flash photolysis of ozone is observed by laser-induced fluorescence (LP-LIF). OH reactivity gives a measure of all OH reactant concentrations, so that potential gaps in the detection of e.g. organic compounds that are relevant for the radical chemistry can be identified (e.g. Nölscher et al., 2012). Unfortunately, the instrument failed in 2014, thus OH reactivity was only measured in one of the two high NO experiments.
Organic compounds were measured by a proton-transfer time-of-flight mass spectrometer (PTR-TOF-MS, Ionicon), which was calibrated to quantify MVK. Methylglyoxal (CHOCOCH3, MGLYOX), and glycolaldehyde (HOCH2CHO) were quantified in one low and one high NO experiment. In the other two experiments, performed in different years, the PTR-TOF-MS was calibrated for MVK, but not for methylglyoxal and glycolaldehyde for all experiments so that these species could not be quantified in all experiments. Acetic acid was detected on the same mass as glycolaldehyde in the PTR-TOF-MS instrument. However, model calculations suggest that the contribution of acetic acid was less than 10 % of the total signal. Therefore, measurements represent glycolaldehyde concentrations reasonably well.
A second PTR-TOF-MS instrument (PTR-3, Ionicon) quantified MVK concentrations in the experiment on 23 May 2017. Measurements of both instruments agreed within 20 %. In addition to direct measurements of MVK concentrations, measurements of the OH reactivity can be used to calculate the MVK concentration that was injected in the experiments because the increase in OH reactivity at that point in time can be attributed to the MVK concentration increase. The comparison with the increase in MVK measurements by the PTR-TOF-MS instrument shows good agreement.
In the experiments in 2017, formaldehyde was measured by the same differential optical absorption spectroscopy (DOAS) instrument that also detects OH radicals in the chamber (Dorn et al., 1995). In the other years, HCHO was measured by a Hantzsch monitor. The 1σ-precision of the formaldehyde measurement of 230 pptv is less than that of the Hantzsch monitor (20 pptv), but it is sufficiently high for the detection of HCHO in the experiments here.
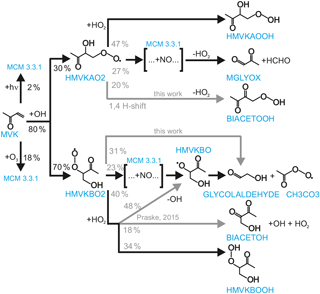
Figure 1Simplified OH oxidation scheme for MVK. Names of compounds are assigned similar to MCM. Modifications to the MCM mechanism (M2) applied in model sensitivity runs M1 and M2 (Table 1) are shown as grey arrows. Reaction yields are calculated for conditions of the experiment with low NO (high ozone concentrations) on 23 May 2017. Grey numbers refer to model run M2.
OH was detected by DOAS (Dorn et al., 1995) in all experiments except for the high NO experiment in 2014. In addition, OH, HO2 and RO2 radicals were measured by laser-induced fluorescence (LIF). The instrument has been described in detail elsewhere (Fuchs et al., 2008, 2011, 2016). OH concentrations measured by LIF in the SAPHIR chamber have been shown to agree with measurements by DOAS in several comparison exercises (e.g. Schlosser et al., 2009; Fuchs et al., 2012). Good agreement was also observed in this work so that significant potential artefacts in the LIF detection scheme as reported for some instruments in the field (Mao et al., 2012; Novelli et al., 2014; Rickly and Stevens, 2018) can be excluded.
HO2 and RO2 are chemically converted to OH by the reaction with NO prior to OH detection by laser-induced fluorescence in the LIF instrument. The conversion of RO2 requires at least two subsequent reactions with NO. First, RO2 is converted through adding NO to HOx in a flow reactor upstream of the fluorescence cell. Added CO in the reactor ensures that the HOx consists predominantly of HO2, which has a small wall loss compared to OH. The reactor is operated at higher pressure (25 hPa) compared to the low-pressure (4 hPa) LIF detection cell (Fuchs et al., 2009). The HOx is sampled from the reactor into the LIF detection cell where HO2 is converted by a large excess of added NO to OH. Operational parameters of the RO2 system are optimized for the efficient detection of RO2 radicals that have a similar reaction rate with NO as methylperoxy radicals. As a consequence, RO2 radicals are not efficiently detected, if their reaction with NO does not directly and quantitatively result in the production of HO2. This is for example the case for the peroxy radical HMVKBO2 (as named in the MCM) that is formed from the reaction of MVK with OH (see below for details). This has to be taken into account, if measured RO2 radicals are compared with model calculations.
The HO2 detection cell consists of a fluorescence cell, in which HO2 reacts with excess NO that is injected behind the inlet nozzle. As shown for several LIF instruments, the HO2 signal can also contain contributions from RO2 radicals that rapidly form HO2 in the reaction with NO (Fuchs et al., 2011; Whalley et al., 2013; Lew et al., 2018). This applies for those RO2 radicals which form an alkoxy radical (RO) in the reaction with NO that rapidly produces HO2 and other products. The interferences from RO2 can be minimised; however, if the instrument is operated with an NO concentration, for which the HO2 to OH conversion efficiency is only approximately less than 10 %. In this case, the RO2 to OH conversion efficiency becomes much smaller for all RO2 species, because the two reactions with NO needed to produce OH limit the overall conversion efficiency. In this study, the HO2 channel of the LIF instrument was operated such that RO2 interferences can be assumed to be negligible.
In one of the experiments (23 May 2017), HO2 was additionally detected by a newly developed chemical ionisation mass spectrometry (CIMS) instrument using Br− as ionisation reagent. HO2 is detected as cluster ion similar to the approaches reported by Veres et al. (2015); Sanchez et al. (2016) using an I− and Br− CIMS, respectively. Details of this new instrument will be presented in a separate publication. HO2 measurements of the CIMS instrument agreed with [HO2] detected by the LIF instrument within 15 %.
Solar radiation was measured outside the chamber using a spectroradiometer. Photolysis frequencies are then calculated by applying a model to transfer outside conditions to conditions inside the chamber (Bohn et al., 2005; Bohn and Zilken, 2005). Latest recommendations for absorption spectra and photolysis yields are used.
Praske et al. (2015)Praske et al. (2015)Karunanandan et al. (2007)Aschmann et al. (1996)Paulson et al. (1998)Praske et al. (2015)Praske et al. (2015)Praske et al. (2015)2.3 Model calculations
Model calculations were performed using the Master Chemical Mechanism in its latest version 3.3.1 (MCM, 2017). A simplified reaction scheme is shown in Fig. 1. The MCM mechanism was modified (MCM*) to take results reported in literature into account and findings in this work. Details are listed in Table 1.
Chamber specific properties were added such as dilution of traces gases due to the replenishment flow. Sources for HONO and HCHO production from the chamber were parameterised as described in previous publications (e.g. Fuchs et al., 2014; Kaminski et al., 2017).
Model calculations were constrained to physical parameters (pressure, temperature, photolysis frequencies and dilution rate of trace gases). A small, constant background OH reactivity of unknown OH reactants that was measured by the OH reactivity instrument after humidification of the chamber air was modelled as an OH reactant that converts OH to HO2. However, the magnitude of this background reactivity was small (<1 s−1) compared to the OH reactivity from MVK during the experiment (>15 s−1) so that it did not affect the chemistry.
Injections of trace gases were modelled as sources during the time of injection, but injected trace gases were not constrained to measured values at later times. [NO], [NO2] and [O3] were only constrained to measurements for the high NOx experiment, because differences between modelled and measured values would have led to significant differences in other observables. No modelling could be performed for one of the high NO experiments (17 May 2017) due to the lack of NOx measurements. No measurements for the reaction rate constants of RO2 species from MVK exist. The sensitivity of model results to a change of the RO2 reaction rate constants, however, is rather small so that their uncertainties could not explain observed model–measurements discrepancies.
Table 2H-migration and HO2 elimination in radicals. Barrier height Eb, reaction energy Ereact and the rate coefficient k at a temperature of 300 K are listed. Arrhenius expressions for a temperature range between 200 and 400 K are available in the Supplement.

a estimated at 0.01 s−1 by Peeters et al. (2009).
2.4 Quantum-chemical calculations
A set of H-migration reactions for the main MVK-derived peroxy radicals was investigated by quantum chemical and theoretical kinetic methodologies. The reactions studied included migration of hydroxyl, α−OH, and methyl H-atoms; direct HO2 elimination forming an enol was also investigated (Table 2).
Several methodologies were applied, as detailed in the supporting information. From these data, the M06-2X/cc-pVTZ rovibrational data (Dunning, 1989; Zhao and Truhlar, 2008), with CCSD(T)/aug-schwartz4(DT) single point energy calculations extrapolated to the basis set limit (Purvis and Bartlett, 1982; Martin, 1996) were selected. All quantum chemical calculations were performed using the Gaussian-09 program suite (Frisch et al., 2010). The high-pressure rate coefficients for each of the elementary processes was then calculated using multi-conformer canonical transition state theory, MC-CTST (Vereecken and Peeters, 2003; Zheng and Truhlar, 2013) based on a rigid rotor harmonic oscillator paradigm, an exhaustive search of the reactants and TS conformers, and asymmetric Eckart tunnelling and WKB zero-curvature (ZCT) tunnelling. For the 1,4- and 1,6-H-shift in HMVKAO2, a large difference between Eckart and ZCT tunnelling was found; the geometric average is reported here (see Supplement).
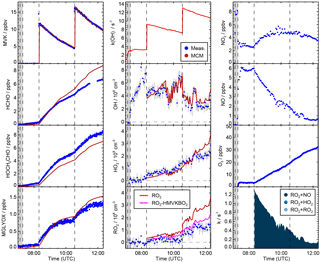
Figure 2Time series of radicals, inorganic and organic species during the MVK photooxidation for the high NO experiment (20 August 2014) together with results from model calculations applying MCM. Dark shaded areas indicate the time before opening the chamber roof and vertical dashed line times when trace gases were injected into the chamber. OH reactivity was not measured during this experiment. NO, NO2 and O3 are constrained to measurements in the model. RO2 loss rates (most lowest right panel) are calculated from modelled HO2, RO2 and NO concentrations. However, contributions from the reactions with RO2 and HO2 or RO2 are too small to be visible. Modelled acetic acid concentrations are small compared to modelled glycolaldehyde concentrations (measured together in the PTR-TOF-MS).
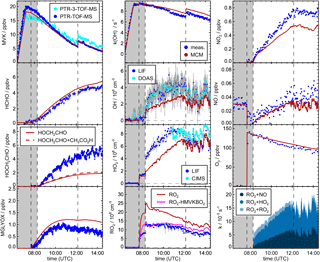
Figure 3Time series of radicals, inorganic and organic species during the MVK photooxidation at low NO (23 May 2017) together with results from model calculations applying MCM. Dark shaded areas indicate the time before opening the chamber roof and vertical dashed line times when trace gases were injected into the chamber. RO2 loss rates (most lowest right panel) are calculated from modelled HO2, RO2 and NO concentrations.
3.1 Product yields
The reaction of OH with MVK leads to the addition of OH to either one of the double-bonded C-atoms so that two different RO2 radical species can be formed (Fig. 1):
Yields are from current recommendations (Atkinson et al., 2006) that are also used in the MCM. The further reaction chain with NO gives glycolaldehyde and an acetylperoxy radical for HMVKBO2 and methylglyoxal, formaldehyde and HO2 for HMVKAO2. In the low NO experiment (23 May 2017), approximately 30 % of the RO2 reacted with NO assuming that the reaction with HO2 was the only competing reaction. In contrast, more than 90 % of RO2 reacted with NO in the high NO experiment. Mixing ratios of the major products formaldehyde, glycolaldeyde and methylglyoxal increased to 6, 8, and 1.3 ppbv, respectively, in the high NO experiment when 13 ppbv MVK was oxidised (Fig. 2). Less of these products was observed in the low NO experiment with 4.5 ppbv formaldehyde, 5 ppbv glycolaldehyde and 1 ppbv methylglyoxal (Fig. 3) when 17 ppbv MVK is oxidised. The smaller concentrations of these products in the low NO case might be expected, because other products can be formed in the competing RO2 reaction channels (Fig. 1).
Tuazon and Atkinson (1989)Grosjean et al. (1993)Praske et al. (2015)Galloway et al. (2011)Tuazon and Atkinson (1989)Grosjean et al. (1993)Praske et al. (2015)Praske et al. (2015)Galloway et al. (2011)Tuazon and Atkinson (1989)Grosjean et al. (1993)Table 3Yields of organic products from photooxidation of MVK by OH from this work and from the literature. Errors of values from this work take into account the accuracy of measurements and precision of the calculation. HCHO is not only produced in the first oxidation step of MVK but also produced in the subsequent oxidation of glycolaldehyde and methylglyoxal. Therefore, the yield can increase over the course of the experiment.
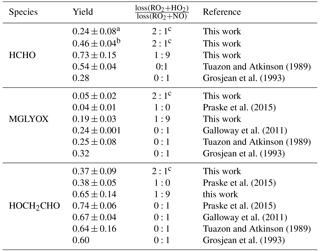
a approximately after 1 h of oxidation. b after 3 h of oxidation. c RO2+RO2 contribution to the total RO2 loss: <20 %.
Because part of the products are oxidised during the experiment, a correction procedure described in detail by Galloway et al. (2011) and Kaminski et al. (2017) is applied, in order to calculate products yields originating from the oxidation of MVK. This correction takes loss of products due to the reaction with OH and photolysis into account and also small production from chamber sources. The relationship between consumed MVK and corrected product concentrations gives the yield of the product species (Table 3, Fig. S3).
For the high NO experiment, when RO2 nearly exclusively reacted with NO, the ratio of product yields for glycolaldehyde (0.65±0.14) and methylglyoxal (0.19±0.03) can be related to the branching ratio of the MVK reaction with OH (Reactions 1 and 1), because these products are formed in either one of the two reaction channels. The values derived from this experiment agree within their uncertainties with studies by Galloway et al. (2011), Tuazon and Atkinson (1989), and Praske et al. (2015) all reporting lower methylglyoxal yields than suggested by current recommendations (Atkinson et al., 2006). This indicates that the branching ratio assumed in the MCM of 0.7 : 0.3 needs to be shifted towards HMVKBO2. Praske et al. (2015) suggests a branching ratio of 0.76 : 0.24. The formaldehyde yield of 0.73±0.15 is higher than the methylglyoxal yield, because formaldehyde is not only a co-product of methylglyoxal in the reaction chain of HMVKAO2 (Fig. 1), but can also be formed from secondary products in the oxidation scheme of MVK. This might also explain why the HCHO yield in this chamber experiment is higher than that in the study by Tuazon and Atkinson (1989). Product yields reported by Grosjean et al. (1993) differ for all three species (largest difference for formaldehyde) from the other studies for unclear reasons.
In the low NO case, other RO2 reaction channels than reaction with NO gain in importance. Lower yields of methylglyoxal (0.05±0.002) and glycolaldehyde (0.37±0.09) compared to the high NO experiment are therefore found in this case. However, the relative decrease of glycolaldehyde is much smaller than that of methylglyoxal. This indicates that glycolaldehyde is also formed from other reaction channels than the reaction of HMVKBO2 with NO. This agrees with results reported by Praske et al. (2015). In that study, a glycolaldehyde yield of 0.38±0.05 was determined in experiments, in which RO2 exclusively reacted with HO2.
3.2 Modelled and measured time series of radical concentrations
Time series of trace gas and radical concentrations are shown together with model calculations using the MCM without modifications for one of the high NO experiments (Fig. 2, 20 August 2014) and for one of the low NO experiments (Fig. 3, 23 May 2017). Although the instrumentation that performed measurements in the experiments were partly different specifically for experiments done in different years, consistent results are obtained. In addition, parameters that are measured by two instruments agree within their combined uncertainties.
MVK (12 ppbv) was injected twice into the sunlit chamber in the high NO experiment (Fig. 2). Approximately half of the MVK reacted away before the second MVK addition was done. The NO mixing ratio decreased over the course of the experiment from nearly 6 to 0.5 ppbv, but was sufficiently high that 90 % of HO2 reacted with NO for most of the time. OH concentrations ranged from (2–4)×106 cm−3 modulated by changes of the OH reactivity and radiation. Model calculations of [OH] agree with measurements within 20 % at all times during the oxidation of MVK. This corresponds to a good description of the measured MVK concentration by the model (deviations less than 5 %) demonstrating that the OH concentration fits the observed oxidation rate of MVK.
HO2 and RO2 radical concentrations were rather small ( cm−3) due to their fast loss in the presence of high NO. Measured and modelled HO2 concentrations show good agreement until the NO mixing ratio decreased below 0.5 ppbv (at 11:00, Fig 2). In the case of RO2, the model yields significantly larger concentrations than measurements. This discrepancy is plausible due to the incomplete conversion of the peroxy radical HMVKBO2 in the pre-reactor. The transformation to HO2 requires more than one NO reaction step and therefore remains incomplete during the transit through the reactor. If the modelled concentration of [HMVKBO2] is subtracted from the total modelled [RO2], good model–measurement agreement is obtained.
Overall, the good agreement between modelled and measured radical concentrations demonstrates that radical chemistry during the oxidation of MVK is well described by state-of-the-art chemical models, if RO2 radicals are mainly lost in the reaction with NO.
In the low NO experiment (Fig. 3), approximately 20 ppbv of MVK was injected, before photooxidation started. As a consequence of the low NO (<40 pptv), RO2 radicals formed in the reaction of MVK with OH not only reacted with NO, but reaction with HO2 and RO2 were competitive. Model calculations using the MCM (Fig. 3) suggest that at least half of the RO2 reacted with HO2 and a smaller fraction (10 to 20 %) with other RO2 radicals.
After nearly 6 h of oxidation, only 4 ppbv MVK was left in the presence of (2–4)×106 cm−3 OH. The amount of MVK that is injected in the model is adjusted to the increase in OH reactivity during the time of injection. The 10 % discrepancy to measured MVK mixing ratios is within the uncertainty of the PTR-TOF-MS calibration. However, the decay of the measured [MVK] is slightly faster than the decay in the model specifically during the first 2 h of oxidation. This corresponds to modelled OH concentrations, which are up to a factor of 2 smaller than measured OH concentrations during this time. At later times of the experiment, measured and modelled [OH] as well as the relative change in [MVK] are matched. Differences between measured and modelled OH concentrations in the first phase of the experiment are accompanied by HO2 concentrations, which are approximately 2×108 cm−3 lower in the model compared to measured values. Measured [HO2] increased from (4–8)×108 cm−3 during the first 2 h. Modelled values match measurements at later times of the experiment like observed for [OH]. The concurrent underestimation of [OH] and [HO2] suggests that a radical source is missing in the model.
In a sensitivity run (MCM*), modifications of reactions that are reported in literature, but do not directly affect the fate of RO2 are implemented:
-
The reaction rate constant of glycolaldehyde with OH is lowered by 20 % following measurements by Karunanandan et al. (2007).
-
Following the results of the product analysis (see above), the branching ratio of Reaction (1) and 1 is changed from 0.3:0.7 to 0.24:0.76 as suggested by Praske et al. (2015).
-
OH yield from ozonolysis of MVK is lowered to 16 % as determined by Aschmann et al. (1996) and Paulson et al. (1998) compared to 36 % assumed in the MCM.
Details are listed in Table 1. Differences with results with the current version of the MCM are rather small (not shown here) so that these modifications do not significantly affect the model–measurement agreement. They are included in the sensitivities model runs shown below.
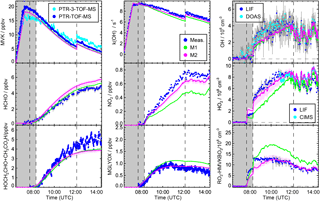
Figure 4Time series of radicals, inorganic and organic species during the MVK photooxidation at low NO (experiment on 23 May 2017). Dark shaded areas indicate the time before opening the chamber roof and vertical dashed line times when trace gases were injected into the chamber. Model sensitivity runs M1 and M2 include modifications listed in Table 1.
4.1 Additional RO2 + HO2 reactions
Product yields indicate that an additional source for glycolaldehyde that is not included in the MCM is required to explain observations in the low NO experiment. This is consistent with chamber experiments by Praske et al. (2015), which were performed under conditions that RO2 exclusively reacted with HO2. In this case, the glycolaldehyde yield is expected to be small, because glycolaldehyde is mainly formed in the subsequent reaction of RO2 with NO (Fig. 1). In that study, also a C4α−diketone (CH3COCOCH2OH, MCM name: BIACETOH) was measured with a yield of 0.14. From these product yields, the authors suggest that there are reaction channels of the HMVKBO2 plus HO2 reaction in addition to the formation of hydroxyperoxides (Praske et al., 2015):
Including Reactions (3)–(3) in the MCM* mechanism (M1 Table 1) results in an improved description of observations for the low NO experiment (Fig. 4). Modelled [OH] agrees with measurements within 20 %. The largest increase of modelled [OH] is obtained 1 to 2 h after starting the oxidation due to the additional radical regeneration from Reactions (3) and (3). The model–measurement agreement is worsened for [RO2] compared to the MCM model run, but still within the uncertainty of the measurement. The additional production of glycolaldehyde from the subsequent chemistry of Reaction (3) brings modelled organic product species into agreement with measurements within 20 % so that all observations except for [HO2] can be explained by the new reactions of HMVKBO2 with HO2. HO2 concentrations, however, are still significantly lower in the model compared to measurements. Results are consistent with experiments by Praske et al. (2015), but a potential underestimation of [HO2] could not be recognised, because HO2 radicals were not measured in their experiments.
4.2 RO2 isomerisation reactions
RO2 isomerisation reactions have been shown to be of importance for the atmospheric fate of RO2 from isoprene (Peeters et al., 2014). Peeters et al. (2009) also suggested from quantum-chemical calculations that HMVKBO2 undergoes a fast 1,5-H shift with subsequent decomposition (Fig. 1 and Table 1):
Here, the possibility of RO2 isomerisation reactions for both major RO2 species formed from the reaction of MVK with OH were investigated in detail by means of quantum-chemical calculations.
Table 2 shows the reaction pathways that were examined for the HMVKAO2 and HMVKBO2 peroxy radicals. More information can be found in the Supplement. For both radicals, 1,5-H-migration of the hydroxyl H-atom is energetically most favourable with a barrier of 22 kcal mol−1. The predicted reaction rate constant remains below s−1, mostly due to limited tunnelling owing to the large reaction endothermicity, and the broad energy barrier protruding less than 2 kcal mol−1 above the reaction products. The concomitant low energy barrier for the reverse H-migration in the product implies that the reaction might be competitive against the alkoxy product decomposition with estimated barriers 4 kcal mol−1 (Vereecken and Peeters, 2009). Most of the other reactions considered are several orders of magnitude slower and can be neglected. The predicted rates for all processes considered remain over an order of magnitude below that required to fit the measured OH, HO2 and glycolaldehyde concentrations so that these H-migration reactions do not have a discernible impact on the MVK oxidation chemistry radical budget or product yields at room temperature. As such, the subsequent chemistry after these reactions was not investigated.
The isomerisation rate constant estimated by Peeters et al. (2009) for Reaction (R1) of 0.01 s−1 is about two orders of magnitude faster than calculated here. This is mainly related to the higher energy barriers found at the levels of theory applied in this work. The small reaction rate constant for this reaction is consistent with the small product yield for methylglyoxal found in the low NO experiment, which would need to be significantly higher than calculated, if Reaction (R1) was competitive with the other RO2 reaction channels. A similar conclusion was drawn from product yields obtained in the study by Praske et al. (2015).
The fastest reaction rate coefficient of RO2 isomerisation reactions calculated here is found to be the HMVKAO2 1,4-H-migration of the hydrogen atoms adjacent to the −OH group, followed by H abstraction at the −OH site by O2 forming HO2 together with a bi-ketone (named BIACETOOH in the MCM):
A reaction rate constant of 0.003 s−1 is calculated making this reaction competitive with the reaction of HMVKAO2 with HO2 and NO in the low NO experiments. Approximately 20 to 30 % of the HVMKAO2 undergoes the 1,4-H shift reaction in this experiment. However, the resulting increase of the HO2 concentration is rather small ( cm−3) because of the small HMVKAO2 yield in the reaction of MVK with OH. This reaction is included in the model sensitivity runs M2.
4.3 Potential additional RO2 reaction channel
A fast conversion of HMVKBO2 to HO2 would be required to fit HO2 measurements. Glycolaldehyde would need to be a co-product to match measured values:
X represents an unknown reaction partner not needed in case of a unimolecular reaction. M2 in Fig. 4 gives the model result, if Reaction (R3) is included in the MCM* in addition to the 1,4-H shift of HMVKAO2 (Reaction R2) and the additional channels of the reaction of HMVKBO2 with HO2 (Reactions 3–3).
In order to fit HO2 and glycolaldehyde concentration measurements, a reaction rate constant of 0.006±0.004 s−1 is required. This reaction rate makes the Reaction (R3) competitive with the reaction of RO2 with NO (reaction rate approximately 0.004 s−1) and HO2 (reaction rate approximately 0.008 s−1). In M2, 40 % of HMVKBO2 reacts with HO2 and 30 % of HMVKBO2 forms directly HO2 and glycolaldehyde in Reaction (R3) for conditions of this experiment. In comparison, 60 % of HMVKBO2 reacts with HO2 in the model run M1. However, the overall effect on the [OH] is similar in both model runs so that modelled [OH] becomes consistent with measurements. This is due to the conversion of HO2 produced in Reaction (R3) to OH. Overall, however, the major difference in the results of M1 and M2 is in the improved model–measurement agreement of [HO2].
Unfortunately, experiments here do not give hints about the exact nature of Reaction (R3). Quantum-chemical calculations (see above) shows that Reaction (R3) cannot be a unimolecular reaction such as H-atom migration, because they are not fast enough to compete with other RO2 reaction channels. Photolysis of RO2 that could results in OH∕HO2 have been observed for acetylperoxy radicals (Cox et al., 1990) and isoprene derived RO2 (Hansen et al., 2017). However, the reaction rate constant of 0.006±0.004 s−1 needed here to explain observations would require an unrealistically high absorption cross section. A reaction partner in Reaction (R3) could also be a RO2 radical. However, in this case products of the HMVKBO2 plus RO2 reaction that are assumed in the MCM would need to be changed according to Reaction (R3) and the reaction rate constant would need to be increased by a large factor of 20 to 50 compared to recommendations for RO2 self-reaction rate constants in order to make this reaction competitive with the other RO2 reaction channels.
4.4 Model–measurement agreement of nitrogen oxide species
So far, only radicals and organic products have been discussed. However, there is also disagreement between measured and modelled NO2 mixing ratios. The NO2 concentration produced by the model is 30 % smaller in the low NO experiment (23 May 2017)) compared to measured values. This discrepancy increases to 40 %, if the OH concentration and therefore the MVK oxidation rate is increased. This is due to the increased production of peroxy radicals, which form peroxy acyl nitrate (PAN) or PAN like species, which act as nitrogen oxide reservoirs. Acetylperoxy radicals forming PAN are mainly produced from HVMKBO2 as a co-product of glycolaldehyde, but another PAN-like species (MCM name PHAN) is additionally produced by the oxidation of glycolaldehyde. If no production of PHAN is assumed, measured and modelled NO2 mixing ratios agree within 100 pptv (M2 in Fig. 4), less than the accuracy of the NOx formation in the chamber. However, also reduction of the production of acetylperoxy radicals could improve the model–measurement agreement. The change in NO and peroxy radical concentrations is rather small, because of the suppression of NO by O3 and the overall small turnover rate of HO2 and NO. More specific experiments concerning the NOx budget would be required to decide, which NOx reservoir species is overestimated by the model.
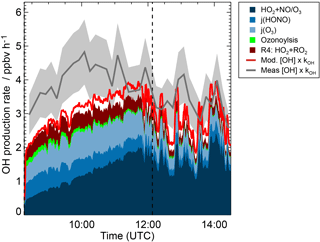
Figure 5OH budget during the experiment at low NO concentration (23 May 2017) for the period, when MVK photooxidation took place. The vertical dashed line indicates when MVK was reinjected into the chamber. Red and blue coloured areas add contributions to the OH production calculated from the model results (M2 sensitivity run in Fig. 4). The contribution RO2 + HO2 refers to the OH production from Reaction (3). In addition to modelled OH production contributions, total OH production calculated from the product of OH concentration and OH reactivity (kOH) is shown. These quantities are either taken from the model results (M2) or from measurements. The coloured grey area gives the uncertainty of the total OH production calculation, if measured OH concentrations and measured OH reactivity are used.
4.5 OH budget analysis
OH is in steady state because of its short lifetime, so that its rates of production and destruction are equal. Therefore, OH reactivity together with OH concentration measurements allows determination of the total OH production rate from only two measured quantities. Under conditions with high NO concentrations, only few chemical reactions are typically controlling the OH production. The dominating process is usually the recycling of HO2 by its reaction with NO. In addition, photolysis of ozone and HONO make significant contributions. For low NO conditions and in the presence of high VOC concentrations, field and chamber experiments often show larger total OH productions rates (derived from measured OH and OH reactivity) than can be explained by the above processes. Under these conditions, other OH sources linked to the degradation of VOCs become relevant.
Figure 5 shows the OH budget for the low NO experiment (23 May 2017) using model results (M2, Table 1) to calculate contributions to the OH production. In addition, total OH production is calculated from measured and modelled OH concentrations and OH reactivity. Results from calculations using either modelled or measured values give similar numbers that agree within the uncertainty of the calculation of 20 %.
The OH production rate is dominated by HO2 recycling reactions and primary OH production (HONO and O3 photolysis). These contributions explain 70 to 80 % of the total OH production during the MVK oxidation. The model would give lower OH production compared to calculations using measurements if Reaction (R3) was not included because HO2 concentrations and therefore OH recycling by HO2 would be underpredicted in this case. This reaction is responsible for approximately half of the HO2 concentration during the first 2 h of MVK oxidation. This demonstrates the importance of including all HO2 sources in models.
Another 10 to 15 % of the total OH production rate is due to the OH formation from the additional HO2 plus RO2 reaction channel suggested by Praske et al. (2015) (Reaction 3). A large number of other OH forming reactions included in the model such as photolysis of hydroxyperoxides fills the remaining gap between these major contributions and the total calculated OH production.
The photooxidation of MVK, one of the major oxidation products of isoprene, was investigated at atmospheric conditions in the simulation chamber SAPHIR. NO was varied from high to low concentrations. For high NO, RO2 is mainly lost in the reaction with NO and current chemical models can describe radical concentrations within 20 %. Product yields of the major oxidation products glycolaldehyde (0.65±0.14) and methylglyoxal (0.19±0.03) are consistent with previous measurements (Tuazon and Atkinson, 1989; Galloway et al., 2011; Praske et al., 2015).
OH radical concentrations are underestimated (maximum factor 2) by the MCM at low NO concentrations (<100 pptv), when other RO2 reaction channels can compete with the reaction of RO2 with NO. At the same time, also HO2 and glycolaldehyde concentrations are smaller in the model compared to measurements. Only part of the model–measurements discrepancies can be explained by findings in recent studies investigating the MVK photooxidation. The higher glycolaldehyde yield is consistent with a study by Praske et al. (2015). The additional channels for the RO2 plus HO2 reaction suggested by these authors can reproduce glycolaldehyde and OH concentrations, but do not explain the model–measurement discrepancy for [HO2].
The possibility of RO2 isomerisation reactions for both major RO2 species formed from the reaction of MVK with OH were investigated in detail by means of quantum-chemical calculations. Additional HO2 can be produced from the 1,4-H shift reaction of HVMKAO2. The reaction rate constant of 0.003 s−1 is competitive with other RO2 reaction channels at low NO conditions. The overall impact, however, is small due to the small HMVKAO2 yield. Other reactions considered here can be neglected for atmospheric conditions. This also includes the isomerisation reaction suggested by Peeters et al. (2009) (Reaction R1). The rate constant for this reaction is about two orders of magnitude smaller than calculated by Peeters et al. (2009) due to the higher energy barriers found at the higher levels of theory applied in this work.
Because HO2 and glycolaldehyde concentrations are underestimated at the same time, a reaction that converts RO2 to HO2 and glycolaldehyde (Reaction R3) would explain observations in these experiments. A reaction rate constant of 0.006±0.004 s−1 is required to bring measured and modelled values into agreement. Unimolecular H-shift reactions are found to be too slow. Alternatively, reaction of HMVKBO2 with RO2 that would produce directly HO2 and glycolaldehyde would explain [HO2] observations and would give similar OH and glycolaldehyde concentrations as the mechanism by Praske et al. (2015). However, more than the product species would need to be different from what is described in the MCM, but also the reaction rate constants would need to be increased by a large factor of 20 to 50 for the HMVKBO2 plus RO2 reaction. More studies will be needed to explore the exact role of HO2 in the MVK oxidation scheme. In addition, open questions remain concerning the fate of nitrogen oxides in the MVK oxidation scheme. The MCM suggests the built-up of nitrogen oxide reservoirs by the formation of PAN and PAN-like species. Experiments here indicate that these reservoirs are less important.
The need for an additional HO2 source was also found in the oxidation of monoterpenes. Field studies, in which OH reactivity was dominated by monoterpenes, showed that models underestimate HO2 and OH concentrations (Kim et al., 2013; Hens et al., 2014). A chamber study investigating the photochemistry of β−pinene found that an additional HO2 source is required to explain observed HO2 and OH and suggested a rearrangement of radical intermediates as explanation (Kaminski et al., 2017).
The impact on the OH recycling efficiency and observed organic products in the MVK oxidation are the same regardless whether OH is directly produced from HO2 plus RO2 like in the Praske et al. (2015) mechanism or if OH is produced from enhanced HO2 as suggested by experiments here. The enhanced OH recycling is demonstrated in this study by the direct quantification of the OH radical concentration during the photochemical oxidation of MVK. Similar as for isoprene (Peeters et al., 2014; Fuchs et al., 2013) and the second major organic product from isoprene oxidation, methacrolein, (Crounse et al., 2011; Fuchs et al., 2014), HOx radicals are faster recycled in the MVK oxidation scheme than previously assumed. For all three species, OH concentrations are found to be a factor of 2 to 3 higher than calculated by models for atmospheric conditions with low NO concentrations. Current state-of-the-art models increased already OH production for isoprene and methacrolein oxidation by including additional reaction pathways. The study here shows that this is also needed for the MVK oxidation scheme.
Data of the experiments in the SAPHIR chamber used in this work is available on the EUROCHAMP data homepage (https://data.eurochamp.org/, last access: April 2018).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-18-8001-2018-supplement.
The authors declare to have no competing interests.
This project has received funding from the European Research Council (ERC)
under the European Union's Horizon 2020 research and innovation programme
(SARLEP grant agreement No. 681529) and from the European Commission (EC)
under the European Union's Horizon 2020 research and innovation programme
(Eurochamp 2020 grant agreement No. 730997). The authors thank the
Forschungszentrum Jülich for travel support under the project “Seed
Money”. Frank N. Keutsch and Alex Zaytsev were supported by the National
Science Foundation (AGS 1628491 and 1628530). Martin Breitenlechner was
supported by the Austrian Science Fund (FWF), Erwin Schrödinger Stipendium,
Grant No. J-3900. The authors thank Thomas Mentel, Yare Baker and Sungah Kang
from Forschungszentrum Jülich for supporting the HO2 CIMS
measurements.
The article processing charges for this open-access
publication were covered by a Research
Centre of the Helmholtz Association.
Edited by: Sergey A. Nizkorodov
Reviewed by: two anonymous referees
Aschmann, S. M., Arey, J., and Atkinson, R.: OH radical formation from the gas-phase reactions of O3 with methacrolein and methyl vinyl ketone, Atmos. Environ., 30, 2939–2943, https://doi.org/10.1016/1352-2310(96)00013-1, 1996. a, b
Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., Jenkin, M. E., Rossi, M. J., Troe, J., and Subcommittee, I.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II – gas phase reactions of organic species, Atmos. Chem. Phys., 6, 3625–4055, https://doi.org/10.5194/acp-6-3625-2006, 2006. a, b
Bohn, B. and Zilken, H.: Model-aided radiometric determination of photolysis frequencies in a sunlit atmosphere simulation chamber, Atmos. Chem. Phys., 5, 191–206, https://doi.org/10.5194/acp-5-191-2005, 2005. a
Bohn, B., Rohrer, F., Brauers, T., and Wahner, A.: Actinometric measurements of NO2 photolysis frequencies in the atmosphere simulation chamber SAPHIR, Atmos. Chem. Phys., 5, 493–503, https://doi.org/10.5194/acp-5-493-2005, 2005. a
Cox, R. A., Munk, J., Nielsen, O. J., Pagsberg, P., and Ratajczak, E.: Ultraviolet absorption spectra and kinetics of acetonyl and acetonylperoxy radicals, Chem. Phys. Lett., 173, 206–210, https://doi.org/10.1016/0009-2614(90)80079-S, 1990. a
Crounse, J. D., Paulot, F., Kjaergaard, H. G., and Wennberg, P. O.: Peroxy radical isomerization in the oxidation of isoprene, Phys. Chem. Chem. Phys., 13, 13607–13613, https://doi.org/10.1039/C1CP21330J, 2011. a, b
Crounse, J. D., Knap, H. C., Ornso, K. B., Jorgensen, S., Paulot, F., Kjaergaard, H. G., and Wennberg, P. O.: On the atmospheric fate of methacrolein: 1. Peroxy radical isomerization following addition of OH and O2, J. Phys. Chem. A, 116, 5756–5762, https://doi.org/10.1021/jp211560u, 2012. a
D'Ambro, E. L., Moller, K. H., Lopez-Hilfiker, F. D., Schobesberger, S., Liu, J., Shilling, J. E., Lee, B. H., Kjaergaard, H. G., and Thornton, J. A.: Isomerization of second-generation isoprene peroxy radicals: epoxide formation and implications for secondary organic aerosol yields, Environ Sci. Technol., 51, 4978–4987, https://doi.org/10.1021/acs.est.7b00460, 2017. a
Dillon, T. J. and Crowley, J. N.: Direct detection of OH formation in the reactions of HO2 with CH3C(O)O2 and other substituted peroxy radicals, Atmos. Chem. Phys., 8, 4877–4889, https://doi.org/10.5194/acp-8-4877-2008, 2008. a
Dorn, H.-P., Brandenburger, U., Brauers, T., and Hausmann, M.: A new in-situ laser long-path absorption instrument for the measurement of tropospheric OH radicals, J. Atmos. Sci., 52, 3373–3380, 1995. a, b
Dunning, T. H.: Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen, J. Chem. Phys., 90, 1007–1023, https://doi.org/10.1063/1.456153, 1989. a
Eurochamp: Database of Atmospheric Simulation Chamber Studies, available at: https://data.eurochamp.org/data-access/chamber-experiments/, last accesss: April 2018.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., A., M. J. J., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Keith, T., Kobayashi, R., Normand, J., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, O., Foresman, J. B., Ortiz, J. V., Cioslowski, J., Fox, D. J., and Pople, J. A.: Gaussian 09, Revision B.01, Gaussian Inc., Wallington CT., 2010. a
Fuchs, H., Hofzumahaus, A., and Holland, F.: Measurement of tropospheric RO2 and HO2 radicals by a laser-induced fluorescence instrument, Rev. Sci. Instrum., 79, 084104, https://doi.org/10.1063/1.2968712, 2008. a
Fuchs, H., Dube, W. P., Lerner, B. M., Wagner, N. L., Williams, E. J., and Brown, S. S.: A sensitive and versatile detector for atmospheric NO2 and NOx based on blue diode laser cavity ring-down spectroscopy, Environ. Sci. Technol., 43, 7831–7836, https://doi.org/10.1021/es902067h, 2009. a
Fuchs, H., Bohn, B., Hofzumahaus, A., Holland, F., Lu, K. D., Nehr, S., Rohrer, F., and Wahner, A.: Detection of HO2 by laser-induced fluorescence: calibration and interferences from RO2 radicals, Atmos. Meas. Tech., 4, 1209–1255, https://doi.org/10.5194/amt-4-1209-2011, 2011. a, b
Fuchs, H., Dorn, H. P., Bachner, M., Bohn, B., Brauers, T., Gomm, S., Hofzumahaus, A., Holland, F., Nehr, S., Rohrer, F., Tillmann, R., and Wahner, A.: Comparison of OH concentration measurements by DOAS and LIF during SAPHIR chamber experiments at high OH reactivity and low NO concentration, Atmos. Meas. Tech., 5, 1611–1626, https://doi.org/10.5194/amt-5-1611-2012, 2012. a
Fuchs, H., Hofzumahaus, A., Rohrer, F., Bohn, B., Brauers, T., Dorn, H.-P., Häseler, R., Holland, F., Kaminski, M., Li, X., Lu, K., Nehr, S., Tillmann, R., Wegener, R., and Wahner, A.: Experimental evidence for efficient hydroxyl radical regeneration in isoprene oxidation, Nat. Geosci., 6, 1023–1026, https://doi.org/10.1038/NGEO1964, 2013. a, b, c
Fuchs, H., Acir, I. H., Bohn, B., Brauers, T., Dorn, H. P., Häseler, R., Hofzumahaus, A., Holland, F., Kaminski, M., Li, X., Lu, K., Lutz, A., Nehr, S., Rohrer, F., Tillmann, R., Wegener, R., and Wahner, A.: OH regeneration from methacrolein oxidation investigated in the atmosphere simulation chamber SAPHIR, Atmos. Chem. Phys., 14, 7895–7908, https://doi.org/10.5194/acp-14-7895-2014, 2014. a, b, c, d
Fuchs, H., Tan, Z., Hofzumahaus, A., Broch, S., Dorn, H. P., Holland, F., Künstler, C., Gomm, S., Rohrer, F., Schrade, S., Tillmann, R., and Wahner, A.: Investigation of potential interferences in the detection of atmospheric ROX radicals by laser-induced fluorescence under dark conditions, Atmos. Meas. Tech., 9, 1431–1447, https://doi.org/10.5194/amt-9-1431-2016, 2016. a
Fuchs, H., Novelli, A., Rolletter, M., Hofzumahaus, A., Pfannerstill, E. Y., Kessel, S., Edtbauer, A., Williams, J., Michoud, V., Dusanter, S., Locoge, N., Zannoni, N., Gros, V., Truong, F., Sarda-Esteve, R., Cryer, D. R., Brumby, C. A., Whalley, L. K., Stone, D., Seakins, P. W., Heard, D. E., Schoemaecker, C., Blocquet, M., Coudert, S., Batut, S., Fittschen, C., Thames, A. B., Brune, W. H., Ernest, C., Harder, H., Muller, J. B. A., Elste, T., Kubistin, D., Andres, S., Bohn, B., Hohaus, T., Holland, F., Li, X., Rohrer, F., Kiendler-Scharr, A., Tillmann, R., Wegener, R., Yu, Z., Zou, Q., and Wahner, A.: Comparison of OH reactivity measurements in the atmospheric simulation chamber SAPHIR, Atmos. Meas. Tech., 10, 4023–4053, https://doi.org/10.5194/amt-10-4023-2017, 2017. a
Galloway, M. M., Huisman, A. J., Yee, L. D., Chan, A. W. H., Loza, C. L., Seinfeld, J. H., and Keutsch, F. N.: Yields of oxidized volatile organic compounds during the OH radical initiated oxidation of isoprene, methyl vinyl ketone, and methacrolein under high-NOX conditions, Atmos. Chem. Phys., 11, 10779–10790, https://doi.org/10.5194/acp-11-10779-2011, 2011. a, b, c, d, e
Grosjean, D., Williams, E. L., and Grosjean, E.: Atmospheric chemistry of isoprene and of its carbonyl products, Environ Sci. Technol., 27, 830–840, https://doi.org/10.1021/es00042a004, 1993. a, b, c, d
Guenther, A. B., Jiang, X., Heald, C. L., Sakulyanontvittaya, T., Duhl, T., Emmons, L. K., and Wang, X.: The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions, Geosci. Model Dev., 5, 1471–1492, https://doi.org/10.5194/gmd-5-1471-2012, 2012. a
Hansen, R. F., Lewis, T. R., Graham, L., Whalley, L. K., Seakins, P. W., Heard, D. E., and Blitz, M. A.: OH production from the photolysis of isoprene-derived peroxy radicals: cross-sections, quantum yields and atmospheric implications, Phys. Chem. Chem. Phys., 19, 2332–2345, https://doi.org/10.1039/C6CP06718B, 2017. a
Hens, K., Novelli, A., Martinez, M., Auld, J., Axinte, R., Bohn, B., Fischer, H., Keronen, P., Kubistin, D., Nölscher, A. C., Oswald, R., Paasonen, P., Petäjä, T., Regelin, E., Sander, R., Sinha, V., Sipilä, M., Taraborrelli, D., Tatum Ernest, C., Williams, J., Lelieveld, J., and Harder, H.: Observation and modelling of HOX radicals in a boreal forest, Atmos. Chem. Phys., 14, 8723–8747, https://doi.org/10.5194/acp-14-8723-2014, 2014. a
Hofzumahaus, A., Rohrer, F., Lu, K., Bohn, B., Brauers, T., Chang, C.-C., Fuchs, H., Holland, F., Kita, K., Kondo, Y., Li, X., Lou, S., Shao, M., Zeng, L., Wahner, A., and Zhang, Y.: Amplified trace gas removal in the troposphere, Science, 324, 1702–1704, https://doi.org/10.1126/science.1164566, 2009. a
Kaminski, M., Fuchs, H., Acir, I. H., Bohn, B., Brauers, T., Dorn, H. P., Häseler, R., Hofzumahaus, A., Li, X., Lutz, A., Nehr, S., Rohrer, F., Tillmann, R., Vereecken, L., Wegener, R., and Wahner, A.: Investigation of the β-pinene photooxidation by OH in the atmosphere simulation chamber SAPHIR, Atmos. Chem. Phys., 17, 6631–6650, https://doi.org/10.5194/acp-17-6631-2017, 2017. a, b, c, d
Karl, M., Dorn, H.-P., Holland, F., Koppmann, R., Poppe, D., Rupp, L., Schaub, A., and Wahner, A.: Product study of the reaction of OH radicals with isoprene in the atmosphere simulation chamber SAPHIR, J. Atmos. Chem., 55, 167–187, https://doi.org/10.1007/s10874-006-9034-x, 2006. a
Karunanandan, R., Hölscher, D., Dillon, T. J., Horowitz, A., Crowley, J. N., Vereecken, L., and Peeters, J.: Reaction of HO with glycolaldehyde HOCH2CHO: Rate coefficients (240–362 K) and mechanism, J. Chem. Phys. A, 111, 897–908, https://doi.org/10.1021/jp0649504, 2007. a, b
Kim, S., Wolfe, G. M., Mauldin, L., Cantrell, C., Guenther, A., Karl, T., Turnipseed, A., Greenberg, J., Hall, S. R., Ullmann, K., Apel, E., Hornbrook, R., Kajii, Y., Nakashima, Y., Keutsch, F. N., DiGangi, J. P., Henry, S. B., Kaser, L., Schnitzhofer, R., Graus, M., and Hansel, A.: Evaluation of HOX sources and cycling using measurement-constrained model calculations in a 2-methyl-3-butene-2-ol (MBO) and monoterpene (MT) dominated ecosystem, Atmos. Chem. Phys., 13, 2031–2044, https://doi.org/10.5194/acp-13-2031-2013, 2013. a
Lelieveld, J., Butler, T. M., Crowley, J. N., Dillon, T. J., Fischer, H., Ganzeveld, L., Harder, H., Lawrence, M. G., Martinez, M., Taraborrelli, D., and Williams, J.: Atmospheric oxidation capacity sustained by a tropical forest, Nature, 452, 737–740, https://doi.org/10.1038/nature06870, 2008. a
Lew, M. M., Dusanter, S., and Stevens, P. S.: Measurement of interferences associated with the detection of the hydroperoxy radical in the atmosphere using laser-induced fluorescence, Atmos. Meas. Tech., 11, 95–108, https://doi.org/10.5194/amt-11-95-2018, 2018. a
Lou, S., Holland, F., Rohrer, F., Lu, K., Bohn, B., Brauers, T., Chang, C. C., Fuchs, H., Häseler, R., Kita, K., Kondo, Y., Li, X., Shao, M., Zeng, L., Wahner, A., Zhang, Y., Wang, W., and Hofzumahaus, A.: Atmospheric OH reactivities in the Pearl River Delta – China in summer 2006: measurement and model results, Atmos. Chem. Phys., 10, 11243–11260, https://doi.org/10.5194/acp-10-11243-2010, 2010. a
Lu, K. D., Rohrer, F., Holland, F., Fuchs, H., Bohn, B., Brauers, T., Chang, C. C., Häseler, R., Hu, M., Kita, K., Kondo, Y., Li, X., Lou, S. R., Nehr, S., Shao, M., Zeng, L. M., Wahner, A., Zhang, Y. H., and Hofzumahaus, A.: Observation and modelling of OH and HO2 concentrations in the Pearl River Delta 2006: a missing OH source in a VOC rich atmosphere, Atmos. Chem. Phys., 12, 1541–1569, https://doi.org/10.5194/acp-12-1541-2012, 2012. a
Mao, J., Ren, X., Brune, W. H., Van Duin, D. M., Cohen, R. C., Park, J. H., Goldstein, A. H., Paulot, F., Beaver, M. R., Crounse, J. D., Wennberg, P. O., DiGangi, J. P., Henry, S. B., Keutsch, F. N., Park, C., Schade, G. W., Wolfe, G. M., and Thornton, J. A.: Insights into hydroxyl measurements and atmospheric oxidation in a California forest, Atmos. Chem. Phys., 12, 8009–8020, https://doi.org/10.5194/acp-12-8009-2012, 2012. a
Martin, J. M. L.: Ab initio total atomization energies of small molecules – towards the basis set limit, Chem. Phys. Lett., 259, 669–678, https://doi.org/10.1016/0009-2614(96)00898-6, 1996. a
MCM: Master Chemical Mechanism, available at: http://mcm.leeds.ac.uk/MCM/, last access: May 2017. a, b, c
Nölscher, A. C., Williams, J., Sinha, V., Custer, T., Song, W., Johnson, A. M., Axinte, R., Bozem, H., Fischer, H., Pouvesle, N., Phillips, G., Crowley, J. N., Rantala, P., Rinne, J., Kulmala, M., Gonzales, D., Valverde-Canossa, J., Vogel, A., Hoffmann, T., Ouwersloot, H. G., Vila-Guerau de Arellano, J., and Lelieveld, J.: Summertime total OH reactivity measurements from boreal forest during HUMPPA-COPEC 2010, Atmos. Chem. Phys., 12, 8257–8270, https://doi.org/10.5194/acp-12-8257-2012, 2012. a
Novelli, A., Hens, K., Tatum Ernest, C., Kubistin, D., Regelin, E., Elste, T., Plass-Dülmer, C., Martinez, M., Lelieveld, J., and Harder, H.: Characterisation of an inlet pre-injector laser-induced fluorescence instrument for the measurement of atmospheric hydroxyl radicals, Atmos. Meas. Tech., 7, 3413–3430, https://doi.org/10.5194/amt-7-3413-2014, 2014. a
Paulson, S. E., Chung, M., Sen, A. D., and Orzechowska, G.: Measurement of OH radical formation from the reaction of ozone with several biogenic alkenes, J. Geophys. Res., 103, 25533–25539, https://doi.org/10.1029/98JD01951, 1998. a, b
Peeters, J., Nguyen, T. L., and Vereecken, L.: HOX radical regeneration in the oxidation of isoprene, Phys. Chem. Chem. Phys., 11, 5935–5939, https://doi.org/10.1039/b908511d, 2009. a, b, c, d, e, f, g
Peeters, J., Müller, J.-F., Stavrakou, T., and Nguyen, V. S.: Hydroxyl radical recycling in isoprene oxidation driven by hydrogen bonding and hydrogen tunneling: The upgraded LIM1 mechanism, J. Phys. Chem. A, 118, 8625–8643, https://doi.org/10.1021/jp5033146, 2014. a, b, c
Praske, E., Crounse, J. D., Bates, K. H., Kurten, T., Kjaergaard, H. G., and Wennberg, P. O.: Atmospheric fate of methyl vinyl ketone: Peroxy radical reactions with NO and HO2, J. Phys. Chem. A, 119, 4562–4572, https://doi.org/10.1021/jp5107058, 2015. a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r, s, t, u, v
Purvis, G. D. and Bartlett, R. J.: A full coupled-cluster singles and doubles model: The inclusion of disconnected triples, J. Chem. Phys., 76, 1910–1918, https://doi.org/10.1063/1.443164, 1982. a
Rickly, P. and Stevens, P. S.: Measurements of a potential interference with laser-induced fluorescence measurements of ambient OH from the ozonolysis of biogenic alkenes, Atmos. Meas. Tech., 11, 1–16, https://doi.org/10.5194/amt-11-1-2018, 2018. a
Rohrer, F., Bohn, B., Brauers, T., Brüning, D., Johnen, F.-J., Wahner, A., and Kleffmann, J.: Characterisation of the photolytic HONO-source in the atmosphere simulation chamber SAPHIR, Atmos. Chem. Phys., 5, 2189–2201, https://doi.org/10.5194/acp-5-2189-2005, 2005. a
Sanchez, J., Tanner, D. J., Chen, D., Huey, L. G., and Ng, N. L.: A new technique for the direct detection of HO2 radicals using bromide chemical ionization mass spectrometry (Br-CIMS): initial characterization, Atmos. Meas. Tech., 9, 3851–3861, https://doi.org/10.5194/amt-9-3851-2016, 2016. a
Schlosser, E., Brauers, T., Dorn, H.-P., Fuchs, H., Häseler, R., Hofzumahaus, A., Holland, F., Wahner, A., Kanaya, Y., Kajii, Y., Miyamoto, K., Nishida, S., Watanabe, K., Yoshino, A., Kubistin, D., Martinez, M., Rudolf, M., Harder, H., Berresheim, H., Elste, T., Plass-Dülmer, C., Stange, G., and Schurath, U.: Technical Note: Formal blind intercomparison of OH measurements: results from the international campaign HOxComp, Atmos. Chem. Phys., 9, 7923–7948, https://doi.org/10.5194/acp-9-7923-2009, 2009. a
St. Clair, J. M., Rivera, J. C., Crounse, J. D., Knap, H. C., Bates, K. H., Teng, A. P., Jørgensen, S., Kjaergaard, H. G., Keutsch, F. N., and Wennberg, P. O.: Kinetics and products of the reaction of the first-generation isoprene hydroxy hydroperoxide (ISOPOOH) with OH, J. Phys. Chem. A, 120, 1441–1451, https://doi.org/10.1021/acs.jpca.5b06532, 2015. a
Tan, D., Faloona, I., Simpas, J. B., Brune, W., Shepson, P. B., Couch, T. L., Summer, A. L., Carroll, M. A., Thornberry, T., Apel, E., Riemer, D., and Stockwell, W.: HOX budget in a deciduous forest: results from the PROPHET summer 1998 campaign, J. Geophys. Res., 106, 24407–24427, https://doi.org/10.1029/2001JD900016, 2001. a
Tuazon, E. C. and Atkinson, R.: A product study of the gas-phase reaction of methyl vinyl ketone with the OH radical in the presence of NOX, Int. J. Chem. Kin., 21, 1141–1152, https://doi.org/10.1002/kin.550211207, 1989. a, b, c, d, e, f
Vereecken, L. and Peeters, J.: The 1,5-H-shift in 1-butoxy: A case study in the rigorous implementation of transition state theory for a multirotamer system, J. Chem. Phys., 119, 5159–5170, https://doi.org/10.1063/1.1597479, 2003. a
Vereecken, L. and Peeters, J.: Decomposition of substituted alkoxy radicals-part I: a generalized structure-activity relationship for reaction barrier heights, Phys. Chem. Chem. Phys., 11, 9062–9074, https://doi.org/10.1039/B909712K, 2009. a
Veres, P. R., Roberts, J. M., Wild, R. J., Edwards, P. M., Brown, S. S., Bates, T. S., Quinn, P. K., Johnson, J. E., Zamora, R. J., and de Gouw, J.: Peroxynitric acid (HO2NO2) measurements during the UBWOS 2013 and 2014 studies using iodide ion chemical ionization mass spectrometry, Atmos. Chem. Phys., 15, 8101–8114, https://doi.org/10.5194/acp-15-8101-2015, 2015. a
Wennberg, P. O., Bates, K. H., Crounse, J. D., Dodson, L. G., McVay, R. C., Mertens, L. A., Nguyen, T. B., Praske, E., Schwantes, R. H., Smarte, M. D., St Clair, J. M., Teng, A. P., Zhang, X., and Seinfeld, J. H.: Gas-Phase reactions of isoprene and its major oxidation products, Chem. Rev., 18, 3337–3390, https://doi.org/10.1021/acs.chemrev.7b00439, 2018. a
Whalley, L. K., Edwards, P. M., Furneaux, K. L., Goddard, A., Ingham, T., Evans, M. J., Stone, D., Hopkins, J. R., Jones, C. E., Karunaharan, A., Lee, J. D., Lewis, A. C., Monks, P. S., Moller, S. J., and Heard, D. E.: Quantifying the magnitude of a missing hydroxyl radical source in a tropical rainforest, Atmos. Chem. Phys., 11, 7223–7233, https://doi.org/10.5194/acp-11-7223-2011, 2011. a
Whalley, L. K., Blitz, M. A., Desservettaz, M., Seakins, P. W., and Heard, D. E.: Reporting the sensitivity of laser-induced fluorescence instruments used for HO2 detection to an interference from RO2 radicals and introducing a novel approach that enables HO2 and certain RO2 types to be selectively measured, Atmos. Meas. Tech., 6, 3425–3440, https://doi.org/10.5194/amt-6-3425-2013, 2013. a
Winiberg, F. A. F., Dillon, T. J., Orr, S. C., Gro ß, C. B. M., Bejan, I., Brumby, C. A., Evans, M. J., Smith, S. C., Heard, D. E., and Seakins, P. W.: Direct measurements of OH and other product yields from the HO2 + CH3C(O)O2 reaction, Atmos. Chem. Phys., 16, 4023–4042, https://doi.org/10.5194/acp-16-4023-2016, 2016. a
Wolfe, G. M., Crounse, J. D., Parrish, J. D., St. Clair, J. M., Beaver, M. R., Paulot, F., Yoon, T., Wennberg, P. O., and Keutsch, F. N.: Photolysis, OH reactivity and ozone reactivity of a proxy for isoprene-derived hydroperoxyenals, Phys. Chem. Chem. Phys., 14, 7276–7286, https://doi.org/10.1039/C2CP40388A, 2012. a
Zhao, Y. and Truhlar, D. G.: The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Account., 120, 215–241, https://doi.org/10.1007/s00214-007-0310-x, 2008. a
Zheng, J. and Truhlar, D. G.: Quantum thermochemistry: Multistructural method with torsional anharmonicity based on a coupled torsional potential, J. Chem. Theory Comput., 9, 1356–1367, https://doi.org/10.1021/ct3010722, 2013. a