the Creative Commons Attribution 3.0 License.
the Creative Commons Attribution 3.0 License.
Molecular distribution and compound-specific stable carbon isotopic composition of dicarboxylic acids, oxocarboxylic acids and α-dicarbonyls in PM2.5 from Beijing, China
Wanyu Zhao
Kimitaka Kawamura
Siyao Yue
Lianfang Wei
Hong Ren
Yu Yan
Mingjie Kang
Linjie Li
Lujie Ren
Senchao Lai
Jie Li
Zifa Wang
This study investigates the seasonal variation, molecular distribution and stable carbon isotopic composition of diacids, oxocarboxylic acids and α-dicarbonyls to better understand the sources and formation processes of fine aerosols (PM2.5) in Beijing. The concentrations of total dicarboxylic acids varied from 110 to 2580 ng m−3, whereas oxoacids (9.50–353 ng m−3) and dicarbonyls (1.50–85.9 ng m−3) were less abundant. Oxalic acid was found to be the most abundant individual species, followed by succinic acid or occasionally by terephthalic acid (tPh), a plastic waste burning tracer. Ambient concentrations of phthalic acid (37.9 ± 27.3 ng m−3) and tPh (48.7 ± 51.1 ng m−3) were larger in winter than in other seasons, illustrating that fossil fuel combustion and plastic waste incineration contribute more to wintertime aerosols. The year-round mass concentration ratios of malonic acid to succinic acid (C3 ∕ C4) were relatively low by comparison with those in other urban aerosols and remote marine aerosols. The values were less than or equal to unity in Beijing, implying that the degree of photochemical formation of diacids in Beijing is insignificant. Moreover, strong correlation coefficients of major oxocarboxylic acids and α-dicarbonyls with nss-K+ suggest that biomass burning contributes significantly to these organic acids and related precursors. The mean δ13C value of succinic acid is the highest among all species, with values of −17.1 ± 3.9 ‰ (winter) and −17.1 ± 2.0 ‰ (spring), while malonic acid is more enriched in 13C than others in autumn (−17.6 ± 4.6 ‰) and summer (−18.7 ± 4.0 ‰). The δ13C values of major species in Beijing aerosols are generally lower than those in the western North Pacific atmosphere, the downwind region, which indicates that stable carbon isotopic compositions of diacids depend on their precursor sources in Beijing. Therefore, our study demonstrates that in addition to photochemical oxidation, high abundances of diacids, oxocarboxylic acids and α-dicarbonyls in Beijing are largely associated with anthropogenic primary emissions, such as biomass burning, fossil fuel combustion and plastic waste burning.
- Article
(2141 KB) -
Supplement
(282 KB) - BibTeX
- EndNote
Haze pollution events are largely characterized by high levels of fine-aerosol particles (PM2.5) and have received considerable public attention in China during the past few years (Cao, 2012; Zhao et al., 2013; Zhang et al., 2014; Sun et al., 2016). PM2.5 influences air quality, visibility, human health, radiative forcing and global climates (Ulbrich et al., 2009; Z. Sun et al., 2013) and is heavily linked to organic aerosols, making up 20–50 % of aerosol mass (Kanakidou et al., 2005) and no less than 90 % in tropical forest areas (Falkovich et al., 2005). Interestingly, large quantities of organic aerosols are water-soluble, resulting in a corresponding proportion of 20 to 75 % of total carbon mass in particles, which originate from incomplete combustion activities (biomass burning: 45–75 %; fossil fuel burning: 20–60 %; Falkovich et al., 2005; Pathak et al., 2011a). Due to their hygroscopic properties, water-soluble organic aerosols (WSOAs) act as an important role in global climate change by influencing solar radiation (Saxena et al., 1995; Facchini et al., 1999).
Homologue series of diacids, oxoacids and α-dicarbonyls comprise a major portion of WSOAs (Kawamura and Ikushima, 1993; Miyazaki et al., 2009). Owing to the existence of two carboxyl groups, diacids are less volatile and highly water-soluble, and they play an important role in acting as cloud condensation nuclei (CCN) to affect the earth's radiative balance (Kanakidou et al., 2005; Andreae and Rosenfeld, 2008). They are widely present in urban (Ho et al., 2007), rural (Kundu et al., 2010a; Cong et al., 2015), marine (Fu et al., 2013), and Arctic atmospheres (Kawamura et al., 1996a). Concentrations of total diacids contribute approximately 1–3 % to the total carbon mass in urban regions and more than 10 % in remote marine atmospheres (Kawamura and Ikushima, 1993; Kawamura et al., 1996b, c). Diacids, keto acids and α-dicarbonyls not only can be directly released from primary emissions like biomass burning (Turnhouse, 1987; Destevou et al., 1998; Schauer et al., 2001; Kundu et al., 2010a), meal cooking (Rogge et al., 1991; Schauer et al., 1999; Zhao et al., 2007), fossil fuel burning (Kawamura and Kaplan, 1987; Rogge et al., 1993) and motor vehicles (Kawamura and Kaplan, 1987; Donnelly et al., 1988), but they are also largely produced by photo-oxidation reactions during atmospheric transport (Kawamura and Yasui, 2005; Kundu et al., 2010b; Cong et al., 2015). The breakdown of relatively long carbon-chain diacids and other related precursors is also one of the key sources of low carbon-number diacids in the atmosphere (Agarwal et al., 2010).
Realizing the physical and chemical characteristics of organic matter is vital for determining the source regions and elucidating the mechanism of evolution of air pollution events. Various measurements have been employed in order to become more familiar with the sources, transformation and long-distance transport of organic compounds, including studies on sugars, unsaturated fatty acids, n-alkanes and n-alcohol, along with aromatic hydrocarbons (Kawamura and Gagosian, 1987; Kawamura et al., 1996a). Zhang et al. (2010) conducted field observations of dicarboxylic acids and pinene oxidation products with a model analysis of the temperature dependencies of emissions, gas–particle partitioning and chemical reactions. Furthermore, the analyses of stable carbon isotope ratios of water-soluble organic acids can be effectively applied to assessing the photochemical aging level and relative contributions of primary emissions to aerosol samples in the atmosphere using the estimated kinetic isotope effect of target compounds with an OH radical (Kawamura and Watanabe, 2004; Wang and Kawamura, 2006). With this approach, it is possible to differentiate the impacts of local sources and long-range transported air masses.
Beijing, the capital of China, is located on the northwest rim of the North China Plain and is surrounded by industrialized areas from the southwest to the east. Emissions from local and regional sources potentially undergo photochemical processes in the course of transport by means of prevalent winds, which influence the atmospheric visibility and quality in Beijing (Xia et al., 2007). Several studies have reported that the source strength in Beijing is characterized by fossil fuel combustion in winter, whereas it is characterized by secondary aerosol formation in summer (G. Wang et al., 2006; Lin et al., 2009; Sun et al., 2015; Ren et al., 2016). Ji et al. (2016) observed increasing photochemical activity in autumn and winter and suggested biomass burning as a substantial pollution factor in Beijing. In addition to the studies on long-term observations of organic aerosols, specific haze pollution episodes occurring in Beijing have been investigated. For example, Huang et al. (2014) concluded that in comparison with secondary sources, primary emissions contributed slightly less to fine particles in haze events at urban locations in China, including Beijing, using both molecular markers and radiocarbon (14C) measurements. To ascertain the influential factors for air quality in Beijing, studies demonstrated that besides motor exhaust, the oxidation pathway of organic species is also critical (Ho et al., 2010, 2015). Although such studies have focused on the characterization of organic aerosols in Beijing at a molecular level, long-term analyses of low molecular weight (LMW) dicarboxylic acids, oxoacids and α-dicarbonyls with their stable carbon isotopic compositions have not been investigated.
To better understand the sources, photochemical processes, and seasonal distributions of organic aerosols in Beijing, PM2.5 samples were collected from September 2013 to July 2014. These samples were analysed for organic carbon (OC), elemental carbon (EC), water-soluble organic carbon (WSOC) and inorganic ions. In addition to reporting the concentrations of and seasonal variations in LMW dicarboxylic acids, oxoacids and α-dicarbonyls, we investigated the seasonal trends in stable carbon isotopic compositions of these water-soluble organic acids. Through these measurements, the contribution of primary emission, long-range transport and the photochemical production of organic matter in Beijing were examined. The effects of air masses on aerosol composition and formation mechanism are also discussed.
2.1 PM2.5 sampling
The sampling site is situated on the rooftop of a building (8 m above ground level) in the Institute of Atmospheric Physics (39∘58′28 N, 116∘22′16 E), which is considered a representative urban site in Beijing (Sun et al., 2012). PM2.5 samples were collected onto preheated (450 ∘C, 6 h) quartz-fibre filters (Pallflex) by using a high-volume air sampler (TISCH, USA) at an airflow rate of 1.0 m3 min−1 for 23 h from September 2013 to July 2014 (n= 65). Field blanks were prepared before, during and after the campaign by putting pre-combusted filters onto the sampler for a few minutes without pumping. After sampling, filters were properly stored at −20 ∘C to avoid microbial degradation of organics and evaporation of semi-volatile components. Beijing is surrounded by Hebei Province and Tianjin Municipality with intensely developed industries (Xia et al., 2007), so the atmospheric visibility and quality in Beijing sometimes seriously deteriorate owing to the substantial primary aerosols from these areas.
2.2 Analytical procedures
Aerosol samples were analysed for diacids and related compounds using a method reported previously (Kawamura, 1993; Kawamura and Ikushima, 1993). In brief, water-soluble organic acids were obtained from ultrasonic extraction for small discs of PM2.5 samples submerged in Milli-Q water three times. Then, the sample extractions were concentrated to dryness and further reacted with 14 % BF3/n-butanol. Finally, the derivatized extracts were dissolved in n-hexane and analysed by a split/splitless Agilent 6980GC/FID equipped with an HP-5 column (0.2 mm × 25 m, 0.5 µm film thickness). The same procedure was used to analyse the field blank filters. Concentrations of the target organic acids in this study were corrected for the field blanks. Furthermore, recoveries of major organic acids of this method were better than 85 %.
2.3 Measurement of isotopic compounds
The determination of stable carbon isotope ratio (δ13C) values for LMW organic acids relative to Pee Dee belemnite (PDB) was analysed using the technique developed previously (Kawamura and Watanabe, 2004). In short, an internal standard (n-alkane C13) was added to derivatized fraction of each sample at the correct proportion. δ13C values of the derivatized dibutyl esters or dibutoxy acetals, measured using GC (HP6890)/isotope ratio mass spectrometer (irMS), were then calculated for diacids, keto acids and α-dicarbonyls based on the isotopic mass balance equation. Each aerosol sample was analysed several times to make sure that the differences for major diacids in δ13C were below 1 ‰ in general. But for a few compounds, the analysis differences were less than 2 ‰.
2.4 Inorganic ions, WSOC, OC and EC measurements
A part of each filter was extracted with 20 mL of Milli-Q water under ultrasonication for 30 minutes and passed through a filter head of 0.22 µm nominal pore size (PVDF, Merck Millipore Ltd). Ion chromatography (ICS-2100) was used to determine the concentrations of cations (Na+, NH, K+, Mg2+ and Ca2+). The separation of cations was accomplished by using an IonPac CS 12A (4 × 250 mm) analytical column, with an eluent flow rate of 1.0 mL min−1. Another ICS-2100 system was used to measure the concentrations of anions (F−, Cl−, NO and SO. The separation of anions was accomplished using an IonPac AS11-HC analytical column. The eluent was 25.0 mM KOH at a flow rate of 1.0 mL min−1. The anions and cations were analysed separately after the extraction solution was divided into two paths.
For the WSOC measurement, 3.14 cm2 of each filter was extracted by Milli-Q water (20 mL). After 15 min sonication, the extraction was measured by a Shimadzu TOC-V CPH total carbon analyzer (Aggarwal and Kawamura, 2008). OC and EC were determined by using thermal optical reflectance (TOR) following the Interagency Monitoring of Protected Visual Environments (IMPROVE) protocol on a DRI Model 2001 thermal/optical carbon analyzer (Chow et al., 2005). The limit of detection (LOD) for the carbon analysis was 0.8 for OC and 0.4 µgC cm−2 for EC, with a precision of greater than 10 % for total carbon (TC). The concentrations of inorganic ions, WSOC and OC ∕ EC reported here are all corrected for the field blanks.
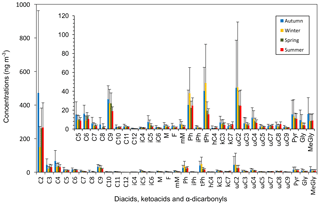
Table 1Concentrations (ng m−3) of dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in PM2.5 samples collected in Beijing from September 2013 to July 2014.
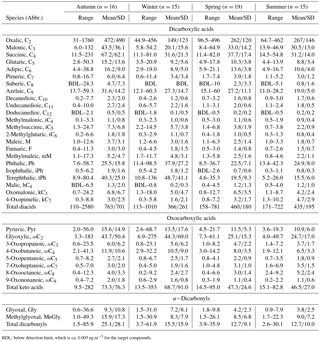
BDL: below detection limit, which is ca. 0.005 ng m−3 for the target compounds.
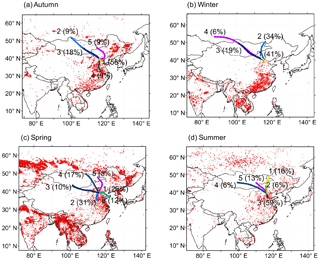
Figure 1Fire spots with typical 5-day air mass backward trajectories (mean clusters) arriving at Beijing for each sampling season. The fire spot data were obtained from the MODIS fire spot website (https://earthdata.nasa.gov/earth-observation-data/near-real-time/firms). The air mass trajectories were drawn using the data obtained by the HYSPLIT4 model from the NOAA ARL website (http://ready.arl.noaa.gov/HYSPLIT.php). The numbers in each panel imply the percentages of hourly trajectories in the sampling season with air mass origins. The arrival height of the air mass backward trajectories was 500 m above sea level.
2.5 Air mass backward trajectories
To better evaluate the influences of air masses from different origins on organic aerosols in Beijing, 5-day backward trajectory analyses with fire spots were performed for each sample from the sampling site at a height of 500 m a.s.l. by using the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT4) model (Rolph et al., 2017). Burning activities in East Asia were illustrated by fire spots, and the datasets were downloaded from the MODIS website (https://earthdata.nasa.gov/earth-observation-data/near-real-time/firms). The backward trajectories were assigned to several major classes on the basis of the prevalent wind direction. And the numbers in each panel implied the percentages of hourly trajectories in the sampling season to better illustrate the air mass origins, as given below in this study (Fig. 1).
3.1 Molecular distribution
Table 1 summarizes the seasonal concentrations of LMW diacids (C2–C12), oxocarboxylic acids (ωC2–ωC9, pyruvic acid), and α-dicarbonyls (C2–C3) in PM2.5 particles in Beijing. Oxalic acid (30.8–1760 ng m−3, average 288 ng m−3) was the predominant individual diacid, showing a peak in autumn and a minimum in winter, whereas its relative abundances (0.39–0.58, average 0.52) to total measured diacids exhibited a maximal and a minimal ratio in summer and wintertime, respectively (Table 3). The predominance of C2 found in this study was coincident with the results of terrestrial and marine particles in previous studies (Kawamura and Yasui, 2005; Ho et al., 2007; Pavuluri et al., 2010; Fu et al., 2013). Among ω-oxocarboxylic acids (ωC2–ωC9), glyoxylic acid (ωC2) was detected as the dominant oxoacid.
Either succinic acid (C4) or occasionally tPh was the second most abundant compound, followed by ωC2 in cold seasons (autumn and winter) or malonic acid (C3) in warm seasons (spring and summer). Total diacids showed the largest abundance in autumn, followed by spring, whereas total oxoacids and α-dicarbonyls both displayed higher levels in cold seasons, especially in autumn (Table 1). The concentrations of individual dicarboxylic acid reduced along with the increase in carbon numbers, but in the range of longer-chain diacids (C6–C12), adipic (C6) and azelaic (C9) acids showed more abundances than other species in the atmosphere throughout the year (Fig. 2).
3.2 Seasonal variation
Seasonal trends in homologue series of diacids and other main species presented three different patterns. The first type, such as oxalic, malonic, succinic, adipic acids and methylglyoxal (MeGly), showed maximum concentrations in autumn with a relatively high abundance in late spring to early summer. The second type showed maximum concentrations in cold seasons (autumn, winter): phthalic, terephthalic and glyoxylic acid peaked in winter, while azelaic acid (C9), glyoxal (Gly), methylmaleic (mM), maleic (M) and fumaric (F) acids peaked in autumn. For the third type, concentrations of methylmalonic (iC4) and 2-methylglutaric (iC6) acids were almost constant throughout the year. These three seasonal patterns indicate different emission sources of the compounds and their precursors and evolution processes of organic aerosols in the atmosphere.
Total concentrations of diacids showed a wide range (110–2580 ng m−3) with an average maximum (763 ng m−3) and minimum (366 ng m−3) in autumn and winter, respectively. These values were comparable to those in Tanzania, East Africa (wet season: 329 ng m−3; dry season: 548 ng m−3 in PM2.5; Mkoma and Kawamura, 2013), slightly lower than those in Tokyo, Japan (726 in June, 682 in July and 438 ng m−3 in November; Kawamura and Yasui, 2005) and a rural site at Gosan, Jeju Island (735 in spring, 784 in summer, 525 in autumn and 500 ng m−3 in winter; Kundu et al., 2010b). The comparisons of the diacids in Beijing with those in other urban cities are presented in Table 2.
Daily variations in diacids and other major organic acids are given in Fig. 3. Oxalic acid has been recognized as the end product that is associated with atmospheric chain reactions of organic species with oxidants (Kawamura and Sakaguchi, 1999). C2 can be generated in abundant quantities by vehicular emission (Kawamura and Kaplan, 1987; Donnelly et al., 1988), biomass burning activities (Turnhouse, 1987; Destevou et al., 1998; Schauer et al., 2001; Kundu et al., 2010a), fossil fuel combustion (Kawamura and Kaplan, 1987; Rogge et al., 1993), and the photo-oxidation of volatile organic compounds and other precursors transported over a long distance (Kawamura and Yasui, 2005; Kundu et al., 2010b). Malonic acid was detected at relatively low concentrations in four sampling periods, with the highest abundance in autumn. The daily variation in malonic acid was almost the same as succinic acid. Concentrations of C4 diacid being in excess of C3 diacid implies that primary emissions contributed more to dicarboxylic acids, a typical pattern that is frequently obtained in aerosols emitted from biomass burning (Kawamura et al., 2013), vehicular exhaust (Ho et al., 2010) and fossil fuel combustion (Ho et al., 2007). The daily variation tendency of C2 resembled that of C3 and C4, indicating that these compounds may have similar photochemical oxidation pathways or emission sources in the atmosphere.
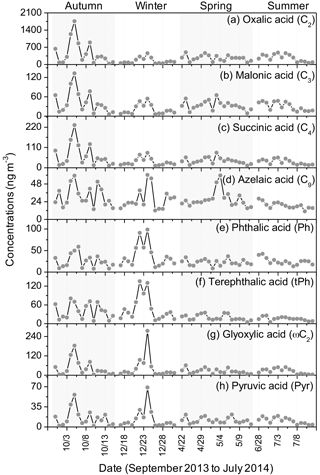
Figure 3Daily variations in the concentrations of selected organic acids in the PM2.5 aerosols in Beijing.
In addition to shorter-chain diacids (C2–C4), azelaic acid (C9) had the highest concentration among the saturated diacids in all seasons (Table 1). C9 is a photochemical oxidation product of unsaturated fatty acids derived from natural biogenic sources such as terrestrial higher plants and sea-to-air emission of marine organics as well as anthropogenic emissions including biomass burning (Kawamura and Gagosian, 1987). Under favourable atmospheric conditions, the photo-oxidation of biogenic unsaturated fatty acids to C9 with oxidants, such as O3, OH and HO2, is inclined to occur in air (Stephanou and Stratigakis, 1993). Additionally, tyre wear debris and traffic exhaust also make contributions to the abundance of LMW fatty acids like C18 : 0, a category of C9 precursors (Rogge et al., 1993).
Azelaic acid was observed in abundance throughout the whole sampling period, while the monthly mean ratios of C9 to total diacids (C9 ∕ Tot) ranged from 0.05 to 0.09, with the highest values in winter (Table 3). Kawamura and Kaplan (1987) reported that C9 can be detected in motor exhaust and may originate from the oxidation of corresponding hydrocarbons, suggesting that dicarboxylic acids are combustion products of normal alkanes in fuels. A great deal of chloride in wintertime is linked to the increased emission of coal incineration in Beijing, particularly under stagnant meteorological conditions that facilitate the formation of particle-phase ammonium chloride (Y. L. Sun et al., 2013). Azelaic acid correlated well with K+ (0.3 ≤ r2≤ 0.4), a tracer for biomass burning (Andreae, 1983), and Cl− (0.4 ≤ r2≤ 0.5) in cold seasons (Fig. S1 in the Supplement), indicating that substantial amounts of C9 may stem from the local and surrounding combustion activities in Beijing.
Ph and tPh are both more abundant in cold seasons than in warm seasons. We found Ph to be the fourth most abundant species in winter (37.9 ± 27.2 ng m−3) and summer (24.9 ± 8.2 ng m−3). Concentration ranges of Ph (7.6–98.5 ng m−3; mean: 31.7 ng m−3) in cold seasons were larger than those (0.08–7.47 ng m−3; mean: 1.76 ng m−3) from Gosan, Jeju Island (Kundu et al., 2010b), but were obviously lower than those (53–278 ng m−3; mean: 150 ng m−3) in urban Xi'an (Y. L. Sun et al., 2013). Phthalic acid is either formed via photochemical pathways of naphthalene or directly released into air by fossil fuel burning and the incomplete combustion of aromatic hydrocarbons in motor vehicles. Moreover, the abundance of Ph may also be caused by increased phthalates emissions from plastic waste burnings in heavily polluted areas in China (Deshmukh et al., 2016). Phthalic acid esters are used as plasticizers in resins and polymers (Simoneit et al., 2005). Therefore, anthropogenic emissions contributed to relatively high concentrations of Ph in PM2.5 in Beijing.
Terephthalic acid (tPh), the second highest abundant diacid in winter (48.7 ± 41.1 ng m−3), showed a pattern in contrast to a previous study that reported Ph as the second most abundant compound (Ho et al., 2010). Terephthalic acid is directly emitted from plastic wastes incinerations in ambient air (Simoneit et al., 2005; Fu et al., 2010; Kawamura and Pavuluri, 2010). High concentrations of tPh observed in winter indicate substantial plastic waste incineration. Another phthalic isomer, isophthalic acid (iPh), was also detected in the samples; concentrations of this isomer had a seasonal pattern similar to those of Ph and tPh throughout the year. However, the concentrations of iPh were the lowest among these isomers.
Oxocarboxylic acids, which are understood as the intermediate products of the oxidation of mono-carboxylic acids, can further be photochemically oxidized to form diacids (Warneck, 2003; Carlton et al., 2007). Concentrations of all keto acids varied from 9.50 to 353 ng m−3 during sampling periods with a maximum (73.3 ± 76.3 ng m−3) in autumn and a minimum (46.5 ± 26.8 ng m−3) in summer. Except for ωC7 and ωC8, the oxoacids showed larger concentrations in cold seasons (autumn and winter), which might be attributed to accumulation under stagnant meteorological conditions. Their concentrations were higher than those in aerosols from Tanzania (60.0 ± 19.0 and 31.0 ± 18.0 ng m−3 in PM2.5 during dry and wet seasons, respectively; Mkoma and Kawamura, 2013) but much lower than these detected in Mangshan, a rural site in Beijing (159 ng m−3 in the daytime, 97.9 ng m−3 in the nighttime; He et al., 2014).
Glyoxylic acid (ωC2) is measured as the most abundant oxoacid, followed by pyruvic (Pyr) and 4-oxobutanoic (ωC4) acids. All of them are important intermediates in photo-oxidation processes for the production of low carbon-number diacids such as C2, C3 and C4 diacids (Hatakeyama et al., 1987). ωC2 and Pyr are more abundant in cold seasons (Table 1) with similar seasonal patterns (Fig. 3g–h). Both correlated well with K+ and Cl− in sampling seasons (Figs. S2–S3), which demonstrate that ωC2 and Pyr originated from common combustion emissions or similar secondary formation pathways. 9-Oxononanoic acid (ωC9), photochemically generated from unsaturated fatty acids (Kawamura and Gagosian, 1987), showed larger concentrations in autumn and winter. This concentration trend was consistent with that of azelaic acid. Additionally, a lower thermal inversion layer, less precipitation and a slower wind speed can enhance the accumulation of organic compounds.
Total concentrations of α-dicarbonyls varied across a wide range (1.50–85.9 ng m−3) and were relatively more abundant in cold seasons (25.1 ± 28.1 in autumn, 15.5 ± 15.9 ng m−3 in winter). The average seasonal concentrations are larger than those at Gosan, Jeju Island (Kundu et al., 2010b). Glyoxal (Gly) and methylglyoxal (MeGly) are semi-volatile gaseous organic precursors that can be produced by the oxidation of isoprene (Zimmermann and Poppe, 1996), monoterpenes (Fick et al., 2004) and other biogenic volatile organic compounds (VOCs; Ervens et al., 2004) and anthropogenic aromatic hydrocarbons (e.g. benzene and toluene; Volkamer et al., 2001). Both carbonyls can form less volatile polar organic acids including Pyr and ωC2 in subsequent oxidation processes, which are key intermediates to produce oxalic acid. Gly and MeGly correlated well with nss-K+ (Gly: 0.3 ≤ r2≤ 0.9, MeGly: 0.3 ≤ r2 ≤ 0.9) throughout the whole year (Fig. S2), whereas they showed good relations with Cl− (Gly: 0.3 ≤ r2≤ 0.8, 0.4 ≤ r2≤ 0.8) in autumn, winter and summer (Fig. S3). Concentrations of these two carbonyls are largely affected by biogenic precursors (e.g. isoprene and monoterpenes) emitted from vegetation and biomass burning activities during entire sampling periods in addition to coal burning and motor exhaust (aromatic hydrocarbons). Low temperature is favourable for the adsorption and condensation of gaseous organic species on existing particles in cold seasons.
3.3 Correlation analysis and seasonal variations in concentration ratios
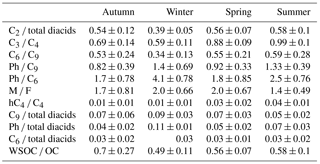
The ratio of oxalic acid to total diacids (C2 ∕ Tot) has been applied to estimate the relative contribution of secondary fraction to atmospheric aerosols during long-range transport. Typically, higher mass concentration ratios are associated with more aged aerosols (Kawamura and Sakaguchi, 1999). The ratios of C2 ∕ Tot were the lowest in winter (0.39 ± 0.05; Table 3), indicating that wintertime organic aerosols may be less aged (Fig. 4a). After the primary emissions of PM2.5 particles from motor vehicles, fossil fuel and biomass burning activities in local regions in winter, aging processes occur during atmospheric transport. In contrast, C2 ∕ Tot ratios are similar in the other three seasons (Table 3). In total, the seasonal mean values of C2 ∕ Tot in this study were lower than the winter ratios (0.8 ± 0.04) in Central Himalayan aerosols owing to the aging of organic compounds occurring in the northerly wind but were close to those in summer (0.5 ± 0.01) due to increased temperature and high wind in the Central Himalayas (Hegde and Kawamura, 2012). Thus, the ratios of C2 ∕ Tot and this seasonal trend indicate that the photochemical formation of dicarboxylic acids is insignificant in urban Beijing.
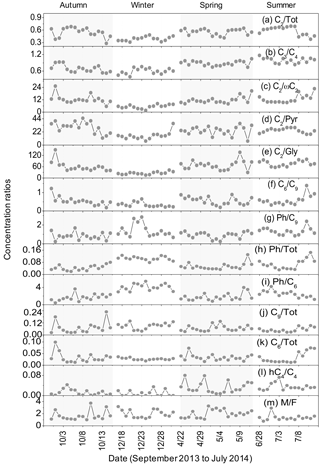
Figure 4Seasonal variations in the concentration ratios of (a) C2 ∕ Tot, (b) C3 ∕ C4, (c) C2 ∕ ωC2, (d) C2 ∕ Pyr, (e) C2 ∕ Gly, (f) C6 ∕ C9, (g) Ph ∕ C9, (h) Ph ∕ Tot, (i) Ph ∕ C6, (j) C9 ∕ Tot, (k) C6 ∕ Tot, (l) hC4 ∕ C4 and (m) M ∕ F in the Beijing aerosols.
Two pathways for the generation of C2, C3 and C4 diacids in air were reported by Kawamura et al. (1996a). On the one hand, C4 diacid can be generated via the photo-oxidation of unsaturated fatty acids from terrestrial higher plants and domestic cooking over continental lands, as well as from phytoplankton emissions over the remote marine regions (Kawamura and Sakaguchi, 1999), and it can subsequently be oxidized to form C3 and C2 diacids (Kawamura and Ikushima, 1993). Typically, concentrations of C2, C4 and C9 diacids showed similar seasonal variation trends (Fig. 3), implying that they were derived from common primary emissions or photochemical processing. Furthermore, C2 ∕ Tot (C2 %) showed strong correlations with C2 ∕ C4 in all four seasons (Fig. S4), indicating the importance of biogenic unsaturated fatty acids, followed by secondary formation ways. On the other hand, aromatic hydrocarbons may be oxidized to produce Gly and ωC2, which are intermediates in the formation of C2 (Kawamura and Ikushima, 1993; Kawamura and Bikkina, 2016). Biogenic and anthropogenic VOCs (e.g. isoprene) can react with oxidants to generate Gly and MeGly in the gas phase. Hydrated α-dicarbonyls can ultimately produce C2 via the photochemical oxidation of Pyr and ωC2 as intermediates (Lim et al., 2005), whereas C3 and C4 diacids cannot be produced in this way.
Therefore, we investigated the importance of these formation pathways by examining the interrelationships between concentration ratios of C2 ∕ Pyr, C2 ∕ ωC2, C2 ∕ Gly and C2 ∕ Tot in this study. Relatively high ratios of C2 ∕ Pyr, C2 ∕ ωC2 and C2 ∕ Gly were observed in autumn, spring and summer (Fig. 4c–e), but their values were much lower than those detected in particulate matters at Gosan, Jeju Island (Kawamura and Yasui, 2005; Kundu et al., 2010b), where the aerosols were relatively aged during long-range transport. Figure S4 shows that strong relationships between C2 ∕ ωC2, C2 ∕ Pyr and C2 ∕ Tot (%) only existed in summer. No correlation was observed between C2 ∕ Gly and C2 ∕ Tot (%). A negative correlation between C2 ∕ ωC2 and C2 ∕ Tot (%) in summer has never been reported previously. These phenomena demonstrate that abundant C2, ωC2, Pyr and Gly were emitted directly from extensive agricultural residue burning, motor vehicles and fossil fuel burning in the studied regions. The supplement of ωC2, Pyr and Gly is faster than their photodegradation to form C2 in air. Only slightly stronger photochemical production of C2 from Pyr was observed in summer.
C3 diacid can be produced as a result of hydrogen abstracted by OH radicals, followed by decarboxylation processing of C4 diacid (Kawamura and Ikushima, 1993). The mass concentration ratio of C3 ∕ C4 is a good indicator for evaluating the contributions of dicarboxylic acids from primary emissions or secondary oxidation production in the atmosphere (Kawamura and Sakaguchi, 1999). Lower C3 ∕ C4 ratios were detected in vehicular exhaust by Kawamura and Kaplan (1987), ranging from 0.25 to 0.44 with an average of 0.35. Less thermally stable C3 diacid can degrade more preferentially to other species rather than remaining stable during incomplete combustion processes.
Figure 4b shows that the C3 ∕ C4 ratios were relatively larger in the warm seasons (spring and summer). However, the temporal trend in the C3 ∕ C4 ratios is relatively flat throughout the sampling year, and most values are less than or equal to unity in Beijing. A low C3 ∕ C4 ratio is associated with the substantial emissions from motor vehicles (Kawamura and Kaplan, 1987). By contrast, the prolonged secondary oxidation of organic matter leads to C3 ∕ C4 values much greater than unity (Kawamura and Ikushima, 1993; Kawamura and Sakaguchi, 1999). The ratios of C3 ∕ C4 reported in this study are lower than that (1-year average of 1.49) in urban Tokyo (Kawamura and Ikushima, 1993) and in the remote Pacific Ocean (average 3; Kawamura and Sakaguchi, 1999), where dicarboxylic acids are largely produced by photochemical reactions. These results demonstrated that in addition to slightly enhanced atmospheric photo-oxidation in summer, incomplete combustions, like motor vehicles and biomass burnings, overwhelmingly contributed to dicarboxylic acids in Beijing.
Phthalic acid (Ph) was one of the most abundant compounds during the sampling period. The seasonal trend in the ratio of phthalic acid to total dicarboxylic acids (Ph ∕ Tot) is shown in Fig. 4h. The Ph ∕ Tot ratios in winter were nearly 2–3 times greater than those in spring and autumn, which implies that phthalic acid is largely emitted by anthropogenic sources in winter, mainly as a result of intensive coal burning for house heating. It is worth noting that the Ph ∕ Tot ratio was also relatively high in summer. A previous study reported that a great amount of naphthalene obtained in Beijing is an important raw material for the substantial formation of phthalic acid (Liu et al., 2007). Therefore, increased ambient temperatures and stronger solar radiation in summertime facilitate the transformation of gaseous polycyclic aromatic hydrocarbons (PAHs; e.g. naphthalene) to produce relative high levels of Ph in Beijing.
C6 and Ph can be formed via secondary oxidation of anthropogenic cyclic olefins (e.g. cyclohexene) and aromatic hydrocarbons, respectively, whereas C9 is mainly produced by photochemical oxidation of biogenic unsaturated fatty acids (Kawamura and Gagosian, 1987; Kawamura and Ikushima, 1993). Thus, the mass concentration ratios of C6 ∕ C9 and Ph ∕ C9 may effectively indicate the source strength of anthropogenic and biogenic emissions to these organic acids.
The seasonally averaged ratios of C6 ∕ Tot, C9 ∕ Tot, C6 ∕ C9 and Ph ∕ C9 are displayed in Table 3. Mean values of C6 ∕ Tot are constantly low in all four seasons, whereas the seasonal ratios of C9 ∕ Tot are the highest (0.09) in winter and the lowest (0.05) in summer, which results in the lowest value of C6 ∕ C9 ratios in winter (0.34 ± 0.13), and are almost constant in the other three seasons. This trend is different from the one detected in the Central Himalayan region (1.07 in winter and 0.56 in summer; Hegde and Kawamura, 2012) and Chennai, India (0.42 for winter and 0.29 for summer; Pavuluri et al., 2010). In contrast, the values of Ph ∕ C9 are relatively high in winter (1.40 ± 0.69) and summer (1.33 ± 0.39), followed by spring (0.92 ± 0.33) and autumn (0.82 ± 0.39); its ratios are obviously lower than the values found in 14 other megacities in China (2.71 for winter and 3.37 for summer; Ho et al., 2007) but are a bit higher than those in Tokyo (0.65 1-year mean value; Kawamura and Ikushima, 1993). From the outcomes discussed above, we concluded that the contribution from anthropogenic emissions was the main source in megacities. Ph ∕ C6 reached the highest values in winter (4.06 ± 0.78) and the lowest ratio in autumn (1.66 ± 0.78). Kawamura and Kaplan (1987) demonstrated that the Ph ∕ C6 ratio from gasoline fuel vehicle (2.05) is lower than that from diesel fuel vehicles (6.58). This phenomenon shows abundances of diacids attributable to more emissions from a gasoline fuel vehicles than diesel burning.
Maleic acid (M), originated predominantly from photochemical oxidation of aromatic hydrocarbons (e.g. benzene and toluene), can be subsequently isomerized to its trans-isomer, fumaric acid (F), under favourable conditions. Lower M ∕ F values have been detected in atmospheric aerosols over the North Pacific Ocean (0.3; Kawamura and Sakaguchi, 1999) as well as at Alert in the high Arctic (ratio range: 0.5–1.0; Kawamura et al., 1996a). The M ∕ F ratios are almost constant in winter (2.0 ± 0.66) and spring (2.0 ± 0.67) and are higher than those in autumn (1.67 ± 0.81) and summer (1.35 ± 0.49). This trend may be associated with substantial amounts of precursors emitted from biomass burning in autumn, fresh aerosols initiated by high-speed winds in spring and enhanced isomerization reaction from M to F under intense solar radiation in summer. The conversion of maleic to fumaric acids can be restrained in polluted environments with minimum weak sunlight (Kundu et al., 2010a). Therefore, M may not be effectively isomerized to F during wintertime in Beijing. Though M ∕ F had the lowest ratio values in summer, dicarboxylic acids and related compounds in Beijing are not seriously subjected to secondary oxidation processes over the whole year when compared to the strength of primary emissions.
Based on field observation, Kawamura and Ikushima (1993) hypothesized that C4 diacid can transform into malic acid (hC4) by means of hydroxylation. The hC4 ∕ C4 ratios were the highest in warm seasons (0.04 ± 0.01 in summer and 0.03 ± 0.02 in spring), which supported this hypothesis. hC4 ∕ C4 ratios in summer are 2–4 times larger than those in cold seasons, similar to the trends observed in Jeju Island, Korea (Kundu et al., 2010b), and urban Tokyo (Kawamura and Ikushima, 1993).
3.4 Comparisons of the mean mass ratios between sampling sites
To assess the emission strength of anthropogenic activities in Beijing, the mean values of C3 ∕ C4 (Fig. 5a), M ∕ F (Fig. 5b), Ph ∕ C9 (Fig. 5c), Ph ∕ Tot (Fig. 5d) and tPh ∕ Tot (Fig. 5e) mass ratios were compared with those at other sampling sites, including inland Xi'an city (Wang et al., 2012); Gosan, Jeju Island (Kundu et al., 2010a); and the western Pacific Ocean (H. Wang et al., 2006). Xi'an, a megacity in the Guanzhong Plain, is located in one of the regions heavily polluted by fossil fuel and biofuel combustion. Atmospheric aerosols at the Gosan site are mixtures of westerly winds from high-latitude regions of Eurasia (Fu et al., 2012b). Marine aerosols over the western Pacific Ocean are a combination of long-range transported continental aerosols and locally emitted marine aerosols (Chen et al., 2013).
Figure 5 presents the global distribution of diagnostic mass ratios of diacids and related compounds. Rather low C3 ∕ C4 ratios were observed in urban aerosols, including Beijing and Xi'an, compared to those aged organic matter collected from Gosan, Jeju Island, and the western Pacific Ocean. Similarly, larger C3 ∕ C4 ratios were obtained in summer than in the other seasons. The same observation in Beijing may be attributable to the enhancement of secondary oxidation that favours the conversion of C4 to C3 in the warm season; however, in that case, the photochemical activity is insignificant compared to the primary emissions. Similar to the C3 ∕ C4 ratios, low M ∕ F ratios indicate the importance of photochemical reaction routes (Kawamura and Sakaguchi, 1999). The mean values of M ∕ F in the Beijing aerosols are larger than or comparable to those reported in Gosan, Jeju Island (spring: 1.4; summer: 0.76; autumn: 1.6; and winter: 2.2), but lower than those obtained in Xi'an aerosols (summer: 2.22; winter: 2.38), indicating that the PM2.5 aerosols in Beijing are mainly linked with regional primary emissions, whereas the photo-isomerization from cis- to trans-isomer is insignificant.
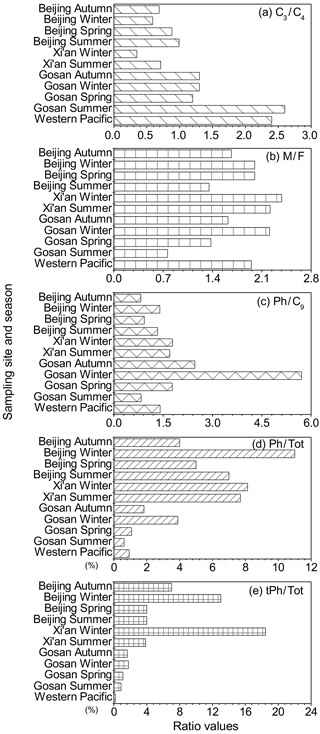
Figure 5Mean mass ratios of (a) C3 ∕ C4, (b) M ∕ F, (c) Ph ∕ C9, (d) Ph ∕ Tot and (e) tPh ∕ Tot from this study compared with those in Xi'an (Wang et al., 2012); Gosan, Jeju Island (Kundu et al., 2010a); and the western Pacific (H. Wang et al., 2006) aerosols.
High Ph ∕ C9 ratios were detected in continental samples owing to a relatively strong contribution from anthropogenic sources to dicarboxylic acids. Slightly larger values of Ph ∕ C9 (on average) were obtained in Xi'an than in Beijing because the air masses in Xi'an were more heavily influenced by intense industrial emissions. Although the values of Ph ∕ C9 in both megacities were higher than those in the western Pacific Ocean, the wintertime Ph ∕ C9 ratios in Gosan were much greater than those in Beijing, which may be caused by the secondary generation of abundant precursors, such as naphthalene, which were transported across a long distance from East Asia.
In this study, we calculated the ratios (%) of Ph and tPh to total diacids to estimate the primary emission strength at different sampling sites. The largest mean mass ratios of Ph ∕ Tot were observed during winter in Beijing, while the values in the other seasons were lower than those observed in Xi'an due to its basin-like topography. For tPh ∕ Tot ratios, the mean values in Beijing were much higher than those in marine areas. However, the average value of tPh ∕ Tot in winter was lower than that in Xi'an. Thus, these comparisons illustrate significant contributions from waste plastic burning and fossil fuel combustion in Beijing during wintertime.
3.5 Source identification by principal component analysis
Previous studies have utilized principal component analysis (PCA) to discriminate between the source apportionments of atmospheric aerosols (Hopke, 1985). In this paper, typical dicarboxylic acids with other major components were chosen for factor analysis. Compounds with common sources or photo-oxidation reactions would be likely to display similarities in mass variations and be assigned to one “factor”. High loadings of variables on the selected species reveal closer links of sources and formation pathways between these compounds (Wu et al., 2015). Here, “total varimax” maximizes the variance of the squared elements in the columns of a factor matrix. The PCA results for dicarboxylic acids and other main components in PM2.5 in Beijing from September 2013 to July 2014 are given in Table 4.
During the whole sampling period, the first factor accounted for 75.2 % of the total variance with high loadings of selected diacids, WSOC and EC (a tracer for incomplete combustion-generated carbon emissions). Typically, the prolonged photochemical oxidation of organics in the atmosphere leads to enhanced concentrations of polar organic matter. WSOC can account for 45–75 % of aerosol carbon mass in biomass burning emissions (Falkovich et al., 2005) and 20–60 % of that in fossil fuel combustion-derived particles (Pathak et al., 2011b). Agricultural waste burning is a serious pollution factor in Beijing (Fig. 1; Viana et al., 2008; Cheng et al., 2014), especially in late June and early October, resulting in substantial organic aerosols (Fu et al., 2012a). In many studies, open-waste burning aerosols are mixed with biomass and fuel burning aerosols (Akagi et al., 2011; Lei et al., 2013). C4, C9, tPh, ωC2, Pyr, Gly and MeGly showed strong correlations in the first factor, implying that burning activities contribute to a large fraction of their concentrations, including biomass burning, biofuel combustion and burning of municipal wastes. For example, the photo-oxidation of p-xylene, a main precursor of the terephthalic acid dimethyl ester, can produce glyoxal (Volkamer et al., 2001; Simoneit et al., 2005; Kawamura and Pavuluri, 2010).
Table 4Results of the principal component analyses for selected diacids and related compounds in PM2.5 in Beijing.
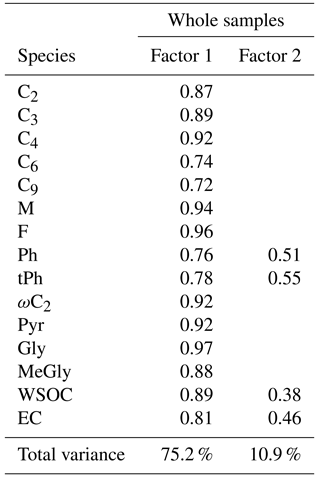
EC and maleic and phthalic acids are clearly associated with other species, indicating that they originate from common mixed sources that are mainly produced by anthropogenic emissions, such as vehicular exhaust, fossil fuel combustion and biomass burning. Aromatic hydrocarbons from incomplete combustions are key materials for maleic and phthalic acids (Kawamura and Sakaguchi, 1999). Both M and Ph showed high abundances under hazy conditions (Mochida et al., 2003).
As for the second factor, Ph, tPh and EC have weak loadings, which seem to originate from motor emissions, fossil fuel combustion and waste plastic burning. WSOC also showed a slight loading in the second factor, which indicates that anthropogenic emissions also contribute to a certain amount of WSOC during the sampling periods.
3.6 Stable carbon isotopic compositions
The systematic differences in stable carbon isotope ratios of diacids and other polar acids were attributable to kinetic isotope fractionation processes in the atmosphere (Hoefs and Hoefs, 1997), while secondary oxidation of these water-soluble organic acids is more influential for diacid carbons to enrich in 13C (Wang and Kawamura, 2006). For example, the relatively short carbon-chain diacids enriched in 13C were ascribed to the kinetic isotopic effect (KIE) for the photochemical breakdown of longer-chain diacids (Anderson et al., 2004; Irei et al., 2006). Lower dicarboxylic acids with enrichment of 13C may be less active to oxidants (e.g. OH radicals). Therefore, the determinations of δ13C values of dicarboxylic acids and related compounds show vital information about the atmospheric aging processes of aerosols derived from local emissions or long-range transport in air.
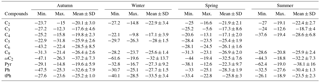
Table 5 presents the stable carbon isotope ratios of major compounds. The mean δ13C values of C2, C3 and C9 were constant among seasons, but those of C4, ωC2, Pyr were smaller in summer than in winter. Because coal is more enriched in 13C than in petroleum fuel (Court et al., 1981; Kawashima and Haneishi, 2012), the 13C enrichment of these organic acids during wintertime may be attributable to the enhanced coal incineration for house heating. Mean δ13C values of malonic acid in autumn and spring were similar to those of succinic acid, suggesting that they may have similar sources or the same secondary formation pathways.
The mean seasonal δ13C values of C9 varying from −25.6 to −26.9 ‰ were smaller than those of C2–C4 diacids. It is noteworthy to state that the depletion of 13C in continental higher plants (C3 plants: −27 ‰) is greater than the particulate organic matter from marine plankton activities (around −20 ‰) (Turekian et al., 2003; Miyazaki et al., 2011). The δ13C values of azelaic acid suggested that unsaturated fatty acids derived from biomass burning in the surrounding areas are a key source of C9 in Beijing.
As mentioned earlier, Ph is mainly formed via the photochemical processes of polycyclic aromatic hydrocarbons, but it can be emitted directly from fossil fuel combustion as well (Kawamura and Kaplan, 1987; Fraser et al., 2003). The largest δ13C value of Ph in winter was linked with its peak concentrations. This finding may be ascribed to the intensity of coal and gasoline combustion in Beijing, especially the stagnant atmospheric conditions that favour the accumulation of organic matter during wintertime (Cao et al., 2011). In general, organic aerosols that are derived from coal and gasoline burning are more enriched in 13C than other emissions, including diesel combustion, aerosols released from C3 plants and secondary organic matter.
For terephthalic acid, the lowest δ13C value of tPh (ave: −33.5 ‰) in winter supports the finding that it is directly emitted from the burning of plastic wastes. Waste burning usually contains many plastics and occurs frequently in open areas without emission control (Kawamura and Pavuluri, 2010), in addition to other local anthropogenic emissions. Lighter δ13C values of major compounds in Beijing than those in the marine and Arctic areas may be explained by more contributions of primary emissions from anthropogenic sources.
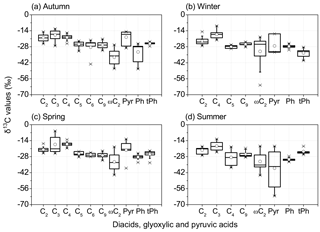
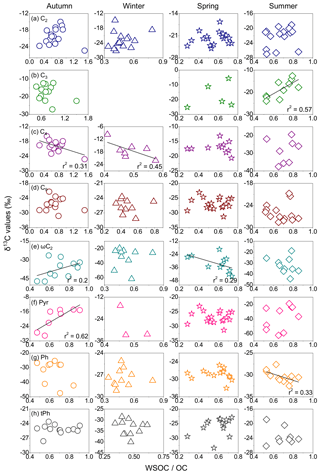
Box plots of stable carbon isotope ratios (δ13C values) are displayed in Fig. 6 for seasonal distributions of diacids, glyoxylic and pyruvic acids in PM2.5. The δ13C values of oxalic acid ranged from −27.2 to −14.8 ‰ in sampling time, with similar seasonal mean δ13C values. Malonic acid was more enriched in 13C than others in autumn (−17.6 ‰) and summer (−18.7 ‰), while succinic acid showed the greatest δ13C value (−17.1 ‰) among all species in winter and spring. A previous study noted that increasing concentrations of oxalic and malonic acids inhibit the growth of total fungi number due to the lower pH, which in turn changes the efficiency of fungi to degrade malonic acid (Côté et al., 2008). Hence, an enrichment of 13C in the remaining malonic acid may be interpreted by the isotopic fractionations occurring in the breakdown of dicarboxylic acids or the photochemical degradation of C3 diacid (Pavuluri and Kawamura, 2012). In this study, the median δ13C values of ωC2 were much lower than those of C2 in all seasons, whereas the δ13C of Pyr showed median values similar to C2 in autumn and spring, which are surprisingly higher than that of C2 in autumn.
3.7 Relations between δ13C values and air mass source areas
In order to further estimate the impacts of air mass source regions on δ13C values of specific compounds, 5-day backward trajectories for each aerosol sample are illustrated in Fig. 1. Data from urban Sapporo (Aggarwal and Kawamura, 2008), Gosan on Jeju Island (Zhang et al., 2016) and remote marine regions (Kawamura and Watanabe, 2004; Wang and Kawamura, 2006) are plotted together with the seasonal mean δ13C values of major species detected in this study (Fig. 7). The largest average δ13C value of oxalic acid was observed in the Gosan samples. The seasonal mean δ13C values of malonic acid in Beijing were higher than those in Sapporo and remote marine areas, but C3 was less enriched in 13C compared to Gosan owing to the degradation of C3 diacid or C2 diacid depleted in 13C. The mean δ13C values of succinic acid are comparable to those in the other three places, except for summer. The mean δ13C values of Pyr in autumn (−19.6 ‰) and spring (−22.3 ‰) were similar to the data in Sapporo (−20.3 ‰) and Gosan (autumn: −19; winter: −22.2; spring: −19.1; summer: −17.6 ‰) aerosols. The δ13C values of ωC2 and Ph in remote marine samples are the highest, followed by those for the Sapporo and Gosan sites and then Beijing. In contrast, the seasonal mean δ13C values of C6 and C9 in Beijing are similar to those in Sapporo and Gosan aerosols but lower than those in marine aerosols.
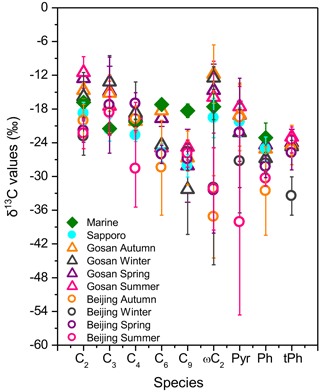
Figure 7Seasonal mean δ13C values of selected diacids and related compounds detected in PM2.5 in Beijing. Data from Sapporo (Aggarwal and Kawamura, 2008); Gosan, Jeju Island (Zhang et al., 2016); and marine (Kawamura and Watanabe, 2004; Wang and Kawamura, 2006) aerosols are also plotted. The bar represents the standard variation (±SD) in the δ13C values.
The air masses in Gosan, Jeju Island, and Sapporo are mixtures of the flows from the mainland of East Asia. The δ13C values illustrate that organic aerosols in Sapporo are formed via the photo-oxidation of precursors originated from anthropogenic and biogenic emissions (e.g. biomass burning) to a large extent, especially C6 and C9; however, the study in Gosan found that aerosol samples are more aged in the western North Pacific rim. Most importantly, particulate organic matter in remote marine areas is intensively aged during long-range transport and is affected by both the sea-to-air emissions and the terrestrial outflows. Moreover, the enrichment in 13C can be regarded as a result of the isotopic fractionation for aged aerosols. Urban aerosols from Beijing, where the air masses are mixed with those originating from Siberia and surrounding areas, are seriously affected by biomass/biofuel burning in the whole year. Compared with the δ13C values in Gosan, Sapporo and remote marine areas, the smaller δ13C values of organic compounds in Beijing may be caused by the different emission strengths of various primary sources.
3.8 Relations between δ13C values and photochemical aging
C2 ∕ Tot ratio is suggested to be a useful tracer to evaluate the aging of atmospheric aerosols (Kawamura and Sakaguchi, 1999). The mean δ13C values of oxalic acid showed the smallest value in winter (−22.9 ‰) and the highest value in autumn (−20.1 ‰), followed by spring (−21.9 ‰; Fig. 8a). Here, we compared the δ13C values of C2 and its concentration changes with the relative abundance of C2 to total diacids (Fig. 8b). The isotopic values of C2 positively correlated with C2 ∕ Tot ratios in autumn (r2= 0.45) and winter (r2= 0.29), suggesting that the production of C2 from the oxidation of precursors can contribute to the increase in δ13C values (Pavuluri et al., 2011). Due to enhanced primary emissions from coal combustion and biomass burning, stagnant atmospheric inversion can favour the accumulation of pollutants. Furthermore, the δ13C values of tPh decreased from autumn to winter, followed by an increase toward summer (Fig. 9). Seasonal δ13C values of tPh decreased with the enhanced ratios of tPh to total diacids (tPh ∕ Tot) in autumn (r2= 0.35) and winter (r2= 0.19), indicating large emissions from municipal waste burning activities in cold seasons, especially in winter.
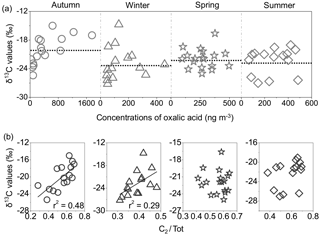
Figure 8(a) Seasonal variations in the stable carbon isotope ratios (δ13C) of C2; (b) correlations between δ13C values of C2 and relative abundances of oxalic acid to total diacids (C2 ∕ Tot) in PM2.5 in Beijing. The black dotted lines represent the average δ13C values.
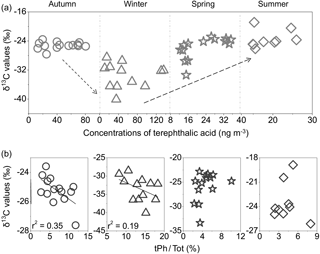
Figure 9(a) Seasonal variations in δ13C of tPh; (b) correlations between the δ13C values of tPh and relative abundances of terephthalic acid to total diacids (tPh ∕ Tot) in PM2.5 in Beijing.
Aged organic aerosols are characterized by a high abundance of polar and water-soluble organic species, leading to high WSOC ∕ OC ratios (Fu et al., 2015). However, in the Beijing samples, the δ13C values of major species (C2, C3, C4, C9, ωC2, Pyr, Ph and tPh) did not show strong relationships with the WSOC ∕ OC ratios in this paper (Fig. 10). The δ13C values of C3 only correlated well with the WSOC ∕ OC ratios in summer (r2= 0.57), in conformity with the variation in C3 ∕ C4 ratios, which illustrates an enhanced degree of photochemical processing of diacids during summertime. The δ13C values of C4 were negatively correlated with WSOC ∕ OC ratios in the cold seasons (autumn: 0.31; winter: 0.45), demonstrating an enrichment of δ13C in C4 with decreasing WSOC ∕ OC ratios. Ph displayed negatively weak correlations in summer, while ωC2 presented weakly positive and negative relations in autumn (0.2) and spring (0.29), respectively. The positive relationship between the δ13C values of Pyr and WSOC ∕ OC in autumn (0.62) suggests that an isotopic enrichment of Pyr increases with high WSOC ∕ OC ratios, which may have resulted in the largest δ13C values of Pyr in autumn. There are no correlations between C2, tPh and C9 with the WSOC ∕ OC ratios in the Beijing samples. Thus, the results discussed above suggest that primary emissions in local regions significantly impact diacids and related compounds in Beijing.
In this study, the molecular distribution and stable carbon isotopic composition of diacids, oxoacids and α-dicarbonyls was determined in fine-aerosol samples (PM2.5) in Beijing over 1 year. Oxalic acid was found to be the most abundant diacid throughout the year. The concentration patterns of major identified organic compounds varied among different seasons. Such differences in molecular compositions were caused by diverse emission strengths of primary emission sources together with photo-oxidation processes in Beijing. Correlation analyses of main oxoacids and α-dicarbonyls with combustion tracers (Cl− and K+) indicate that ωC2, Pyr, Gly and MeGly were mostly affected by biogenic combustions in whole sampling year, with a significant contribution of fossil fuel combustion in winter. The variations in the C3 ∕ C4 ratios were relatively minor during the 1-year observation, with most values less than or equal to unity, which is associated with substantial emissions from vehicular exhausts. Higher ratios of Ph ∕ Tot and tPh ∕ Tot were observed in winter, indicating strong influences of fossil fuel combustion and the burning of plastic waste.
Larger δ13C values obtained in lower carbon-number diacids are mainly interpreted as isotopic fractionations due to the decomposition of longer-chain dicarboxylic acids and related precursors. Although oxalic acid has been regarded as a final product of the photo-oxidation of homologues diacids and related components like Pyr, ωC2 and α-dicarbonyls in the atmosphere, succinic acid showed the largest δ13C value (−17.1 ‰) among all the species in winter and spring, while malonic acid was more enriched in 13C than others in autumn (−17.6 ‰) and summer (−18.7 ‰). The less negative δ13C value of malonic acid may be interpreted by the isotopic fractionations occurring in the breakdown of diacids or photochemical degradation of C3 diacid.
On the basis of the weak correlations of C2 ∕ Tot and WSOC ∕ OC with seasonal δ13C values of major species, the results of the principal component analysis, and the comparison of δ13C values in Beijing with those in urban and remote marine aerosols, we can conclude that photochemical production of dicarboxylic acids and related compounds in the Beijing aerosols slightly increases in summer. But the high abundance of diacids and related polar acids in fine aerosols in Beijing are mainly associated with anthropogenic primary emissions such as biomass burning, fossil fuel combustion and plastic burning. Further studies are needed to interpret the detailed mechanism of the enrichment of the δ13C values of C3 and C4 diacids and to better evaluate the impact of micro-biological degradation along with contact-induced chemical changes on the aerosol chemistry in Beijing.
The data in this study are available upon request from the corresponding author (fupingqing@tju.edu.cn).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-18-2749-2018-supplement.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Regional transport and transformation of air pollution in eastern China”. It is not associated with a conference.
This study was supported by the National Natural Science Foundation of China
(grant nos. 41625014, 41475117, 41571130024 and 91543205) and the Strategic
Priority Research Program (B) of the Chinese Academy of Sciences (grant no.
XDB05030306).
Edited by: Hang Su
Reviewed by: three anonymous referees
Agarwal, S., Aggarwal, S. G., Okuzawa, K., and Kawamura, K.: Size distributions of dicarboxylic acids, ketoacids, α-dicarbonyls, sugars, WSOC, OC, EC and inorganic ions in atmospheric particles over Northern Japan: implication for long-range transport of Siberian biomass burning and East Asian polluted aerosols, Atmos. Chem. Phys., 10, 5839–5858, https://doi.org/10.5194/acp-10-5839-2010, 2010.
Aggarwal, S. G. and Kawamura, K.: Molecular distributions and stable carbon isotopic compositions of dicarboxylic acids and related compounds in aerosols from Sapporo, Japan: Implications for photochemical aging during long-range atmospheric transport, J. Geophys. Res., 113, D14301, https://doi.org/10.1029/2007JD009365, 2008.
Akagi, S. K., Yokelson, R. J., Wiedinmyer, C., Alvarado, M. J., Reid, J. S., Karl, T., Crounse, J. D., and Wennberg, P. O.: Emission factors for open and domestic biomass burning for use in atmospheric models, Atmos. Chem. Phys., 11, 4039–4072, https://doi.org/10.5194/acp-11-4039-2011, 2011.
Anderson, R. S., Huang, L., Iannone, R., Thompson, A. E., and Rudolph, J.: Carbon kinetic isotope effects in the gas phase reactions of light alkanes and ethene with the OH radical at 296 ± 4 K, J. Phys. Chem. A, 108, 11537–11544, 2004.
Andreae, M. and Rosenfeld, D.: Aerosol–cloud–precipitation interactions. Part 1. The nature and sources of cloud-active aerosols, Earth-Sci. Rev., 89, 13–41, 2008.
Andreae, M. O.: Soot carbon and excess fine potassium: long-range transport of combustion-derived aerosols, Science, 220, 1148–1151, 1983.
Cao, J.: Pollution status and control strategies of PM2. 5 in China, J. Earth Environ., 3, 1030–1036, 2012.
Cao, J.-J., Chow, J. C., Tao, J., Lee, S.-C., Watson, J. G., Ho, K.-F., Wang, G.-H., Zhu, C.-S., and Han, Y.-M.: Stable carbon isotopes in aerosols from Chinese cities: Influence of fossil fuels, Atmos. Environ., 45, 1359–1363, 2011.
Carlton, A. G., Turpin, B. J., Altieri, K. E., Seitzinger, S., Reff, A., Lim, H.-J., and Ervens, B.: Atmospheric oxalic acid and SOA production from glyoxal: Results of aqueous photooxidation experiments, Atmos. Environ., 41, 7588–7602, 2007.
Chen, J., Kawamura, K., Liu, C.-Q., and Fu, P. Q.: Long-term observations of saccharides in remote marine aerosols from the western North Pacific: A comparison between 1990–1993 and 2006–2009 periods, Atmos. Environ., 67, 448–458, 2013.
Cheng, C., Wang, G., Zhou, B., Meng, J., Li, J., Cao, J., and Xiao, S.: Comparison of dicarboxylic acids and related compounds in aerosol samples collected in Xi'an, China during haze and clean periods, Atmos. Environ., 81, 443–449, 2013.
Cheng, Y., Engling, G., He, K.-B., Duan, F.-K., Du, Z.-Y., Ma, Y.-L., Liang, L.-L., Lu, Z.-F., Liu, J.-M., and Zheng, M.: The characteristics of Beijing aerosol during two distinct episodes: Impacts of biomass burning and fireworks, Environ. Pollut., 185, 149–157, 2014.
Chow, J. C., Watson, J. G., Chen, L.-W. A., Paredes-Miranda, G., Chang, M.-C. O., Trimble, D., Fung, K. K., Zhang, H., and Zhen Yu, J.: Refining temperature measures in thermal/optical carbon analysis, Atmos. Chem. Phys., 5, 2961–2972, https://doi.org/10.5194/acp-5-2961-2005, 2005.
Cong, Z. Y., Kawamura, K., Kang, S. C., and Fu, P. Q.: Penetration of biomass-burning emissions from South Asia through the Himalayas: new insights from atmospheric organic acids, Sci. Rep., 5, 9580, https://doi.org/10.1038/srep09580, 2015.
Côté, V., Kos, G., Mortazavi, R., and Ariya, P. A.: Microbial and “de novo” transformation of dicarboxylic acids by three airborne fungi, Sci. Total Environ., 390, 530–537, 2008.
Court, J., Goldsack, R., Ferrari, L., and Polach, H.: The use of carbon isotopes in identifying urban air particulate sources, Clean Air, 15, 6–11, 1981.
Deshmukh, D. K., Kawamura, K., Lazaar, M., Kunwar, B., and Boreddy, S. K. R.: Dicarboxylic acids, oxoacids, benzoic acid, α-dicarbonyls, WSOC, OC, and ions in spring aerosols from Okinawa Island in the western North Pacific Rim: size distributions and formation processes, Atmos. Chem. Phys., 16, 5263–5282, https://doi.org/10.5194/acp-16-5263-2016, 2016.
Destevou, P. O., Montenat, C., Ladure, F., and Dautrey, L. P.: Tectonic-sedimentary evolution of the Eastern Prebetic domain (Spain) during the Miocene, Comptes Rendus De L Academie Desences Serie II, 87, 111–123, 1998.
Donnelly, T. H., Shergold, J. H., and Southgate, P. N.: Anomalous geochemical signals from phosphatic middle Cambrian rocks in the southern Georgina basin, Australia, Sedimentology, 35, 549–570, 1988.
Ervens, B., Feingold, G., Frost, G. J., and Kreidenweis, S. M.: A modeling study of aqueous production of dicarboxylic acids: 1. Chemical pathways and speciated organic mass production, J. Geophys. Res.-Atmos., 109, D15205, https://doi.org/10.1029/2003JD004387, 2004.
Facchini, M. C., Mircea, M., Fuzzi, S., and Charlson, R. J.: Cloud albedo enhancement by surface-active organic solutes in growing droplets, Nature, 401, 257–259, 1999.
Falkovich, A. H., Graber, E. R., Schkolnik, G., Rudich, Y., Maenhaut, W., and Artaxo, P.: Low molecular weight organic acids in aerosol particles from Rondônia, Brazil, during the biomass-burning, transition and wet periods, Atmos. Chem. Phys., 5, 781–797, https://doi.org/10.5194/acp-5-781-2005, 2005.
Fick, J., Nilsson, C., and Andersson, B.: Formation of oxidation products in a ventilation system, Atmos. Environ., 38, 5895–5899, 2004.
Fraser, M., Cass, G., and Simoneit, B.: Air quality model evaluation data for organics. 6. C3-C24 organic acids, Environ. Sci. Technol., 37, 446-453, 2003.
Fu, P., Kawamura, K., Usukura, K., and Miura, K.: Dicarboxylic acids, ketocarboxylic acids and glyoxal in the marine aerosols collected during a round-the-world cruise, Mar. Chem., 148, 22–32, 2013.
Fu, P. Q., Kawamura, K., Pavuluri, C. M., Swaminathan, T., and Chen, J.: Molecular characterization of urban organic aerosol in tropical India: contributions of primary emissions and secondary photooxidation, Atmos. Chem. Phys., 10, 2663–2689, https://doi.org/10.5194/acp-10-2663-2010, 2010.
Fu, P. Q., Kawamura, K., Chen, J., Li, J., Sun, Y. L., Liu, Y., Tachibana, E., Aggarwal, S. G., Okuzawa, K., Tanimoto, H., Kanaya, Y., and Wang, Z. F.: Diurnal variations of organic molecular tracers and stable carbon isotopic composition in atmospheric aerosols over Mt. Tai in the North China Plain: an influence of biomass burning, Atmos. Chem. Phys., 12, 8359–8375, https://doi.org/10.5194/acp-12-8359-2012, 2012a.
Fu, P. Q., Kawamura, K., Kobayashi, M., and Simoneit, B. R. T.: Seasonal variations of sugars in atmospheric particulate matter from Gosan, Jeju Island: Significant contributions of airborne pollen and Asian dust in spring, Atmos. Environ., 55, 234–239, 2012b.
Fu, P. Q., Kawamura, K., Chen, J., Qin, M. Y., Ren, L. J., Sun, Y. L., Wang, Z. F., Barrie, L. A., Tachibana, E., Ding, A. J., and Yamashita, Y.: Fluorescent water-soluble organic aerosols in the High Arctic atmosphere, Sci. Rep.-UK, 5, 9845, https://doi.org/10.1038/srep09845, 2015.
Hatakeyama, S., Ohno, M., Weng, J., Takagi, H., and Akimoto, H.: Mechanism for the formation of gaseous and particulate products from ozone-cycloalkene reactions in air, Environ. Sci. Technol., 21, 52–57, 1987.
He, N., Kawamura, K., Okuzawa, K., Pochanart, P., Liu, Y., Kanaya, Y., and Wang, Z.: Diurnal and temporal variations of water-soluble dicarboxylic acids and related compounds in aerosols from the northern vicinity of Beijing: Implication for photochemical aging during atmospheric transport, Sci. Total Environ., 499, 154–165, 2014.
Hegde, P. and Kawamura, K.: Seasonal variations of water-soluble organic carbon, dicarboxylic acids, ketocarboxylic acids, and α-dicarbonyls in Central Himalayan aerosols, Atmos. Chem. Phys., 12, 6645–6665, https://doi.org/10.5194/acp-12-6645-2012, 2012.
Ho, K., Lee, S., Ho, S. S. H., Kawamura, K., Tachibana, E., Cheng, Y., and Zhu, T.: Dicarboxylic acids, ketocarboxylic acids, α dicarbonyls, fatty acids, and benzoic acid in urban aerosols collected during the 2006 Campaign of Air Quality Research in Beijing (CAREBeijing-2006), J. Geophys. Res.-Atmos., 115, D19312, https://doi.org/10.1029/2009JD013304, 2010.
Ho, K. F., Cao, J. J., Lee, S. C., Kawamura, K., Zhang, R. J., Chow, J. C., and Watson, J. G.: Dicarboxylic acids, ketocarboxylic acids, and dicarbonyls in the urban atmosphere of China, J. Geophys. Res., 112, D22S27, https://doi.org/10.1029/2006JD008011, 2007.
Ho, K. F., Huang, R.-J., Kawamura, K., Tachibana, E., Lee, S. C., Ho, S. S. H., Zhu, T., and Tian, L.: Dicarboxylic acids, ketocarboxylic acids, α-dicarbonyls, fatty acids and benzoic acid in PM2.5 aerosol collected during CAREBeijing-2007: an effect of traffic restriction on air quality, Atmos. Chem. Phys., 15, 3111–3123, https://doi.org/10.5194/acp-15-3111-2015, 2015.
Hoefs, J. and Hoefs, J.: Stable isotope geochemistry, Springer, 17, 102–107, 1997.
Hopke, P. K.: Receptor modeling in environmental chemistry, John Wiley & Sons, https://doi.org/10.1016/S0003-2670(00)81807-3, 1985.
Huang, R.-J., Zhang, Y., Bozzetti, C., Ho, K.-F., Cao, J.-J., Han, Y., Daellenbach, K. R., Slowik, J. G., Platt, S. M., and Canonaco, F.: High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 514, 218–222, 2014.
Irei, S., Huang, L., Collin, F., Zhang, W., Hastie, D., and Rudolph, J.: Flow reactor studies of the stable carbon isotope composition of secondary particulate organic matter generated by OH-radical-induced reactions of toluene, Atmos. Environ., 40, 5858–5867, 2006.
Ji, D., Zhang, J., He, J., Wang, X., Pang, B., Liu, Z., Wang, L., and Wang, Y.: Characteristics of atmospheric organic and elemental carbon aerosols in urban Beijing, China, Atmos. Environ., 125, 293–306, 2016.
Jung, J., Tsatsral, B., Kim, Y. J., and Kawamura, K.: Organic and inorganic aerosol compositions in Ulaanbaatar, Mongolia, during the cold winter of 2007 to 2008: Dicarboxylic acids, ketocarboxylic acids, and α-dicarbonyls, J. Geophys. Res., 115, https://doi.org/10.1029/2010jd014339, 2010.
Kanakidou, M., Seinfeld, J. H., Pandis, S. N., Barnes, I., Dentener, F. J., Facchini, M. C., Van Dingenen, R., Ervens, B., Nenes, A., Nielsen, C. J., Swietlicki, E., Putaud, J. P., Balkanski, Y., Fuzzi, S., Horth, J., Moortgat, G. K., Winterhalter, R., Myhre, C. E. L., Tsigaridis, K., Vignati, E., Stephanou, E. G., and Wilson, J.: Organic aerosol and global climate modelling: a review, Atmos. Chem. Phys., 5, 1053–1123, https://doi.org/10.5194/acp-5-1053-2005, 2005.
Kawamura, K.: Identification of C2-C10. omega.-oxocarboxylic acids, pyruvic acid, and C2-C3. alpha.-dicarbonyls in wet precipitation and aerosol samples by capillary GC and GC/MS, Anal. Chem., 65, 3505–3511, 1993.
Kawamura, K. and Bikkina, S.: A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources and transformation, Atmos. Res., 170, 140–160, 2016.
Kawamura, K. and Gagosian, R.: Implications of ω-oxocarboxylic acids in the remote marine atmosphere for photo-oxidation of unsaturated fatty acids, Nature, 325, 330–332, 1987.
Kawashima, H. and Haneishi, Y.: Effects of combustion emissions from the Eurasian continent in winter on seasonal δ13C of elemental carbon in aerosols in Japan, Atmos. Environ., 46, 568–579, 2012.
Kawamura, K. and Ikushima, K.: Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere, Environ. Sci. Technol., 27, 2227–2235, 1993.
Kawamura, K. and Kaplan, I. R.: Motor exhaust emissions as a primary source for dicarboxylic acids in Los Angeles ambient air, Environ. Sci. Technol., 21, 105–110, 1987.
Kawamura, K. and Pavuluri, C. M.: New Directions: Need for better understanding of plastic waste burning as inferred from high abundance of terephthalic acid in South Asian aerosols, Atmos. Environ., 44, 5320–5321, 2010.
Kawamura, K. and Sakaguchi, F.: Molecular distributions of water soluble dicarboxylic acids in marine aerosols over the Pacific Ocean including tropics, J. Geophys. Res.-Atmos., 104, 3501–3509, 1999.
Kawamura, K. and Watanabe, T.: Determination of stable carbon isotopic compositions of low molecular weight dicarboxylic acids and ketocarboxylic acids in atmospheric aerosol and snow samples, Anal. Chem., 76, 5762–5768, 2004.
Kawamura, K. and Yasui, O.: Diurnal changes in the distribution of dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban Tokyo atmosphere, Atmos. Environ., 39, 1945–1960, 2005.
Kawamura, K., Kasukabe, H., and Barrie, L. A.: Source and reaction pathways of dicarboxylic acids, ketoacids and dicarbonyls in arctic aerosols: One year of observations, Atmos. Environ., 30, 1709–1722, 1996a.
Kawamura, K., Seméré, R., Imai, Y., Fujii, Y., and Hayashi, M.: Water soluble dicarboxylic acids and related compounds in Antarctic aerosols, J. Geophys. Res.-Atmos., 101, 18721–18728, 1996b.
Kawamura, K., Steinberg, S., and Kaplan, I. R.: Concentrations of monocar ylic and dicar ylic acids and aldehydes in southern California wet precipitations: Comparison of urban and nonurban samples and compositional changes during scavenging, Atmos. Environ., 30, 1035–1052, 1996c.
Kawamura, K., Tachibana, E., Okuzawa, K., Aggarwal, S. G., Kanaya, Y., and Wang, Z. F.: High abundances of water-soluble dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in the mountaintop aerosols over the North China Plain during wheat burning season, Atmos. Chem. Phys., 13, 8285–8302, https://doi.org/10.5194/acp-13-8285-2013, 2013.
Kundu, S., Kawamura, K., Andreae, T. W., Hoffer, A., and Andreae, M. O.: Molecular distributions of dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in biomass burning aerosols: implications for photochemical production and degradation in smoke layers, Atmos. Chem. Phys., 10, 2209–2225, https://doi.org/10.5194/acp-10-2209-2010, 2010a.
Kundu, S., Kawamura, K., and Lee, M.: Seasonal variations of diacids, ketoacids, and α-dicarbonyls in aerosols at Gosan, Jeju Island, South Korea: Implications for sources, formation, and degradation during long-range transport, J. Geophys. Res.-Atmos., 115, D19307, https://doi.org/10.1029/2010JD013973, 2010b.
Lei, W., Li, G., and Molina, L. T.: Modeling the impacts of biomass burning on air quality in and around Mexico City, Atmos. Chem. Phys., 13, 2299–2319, https://doi.org/10.5194/acp-13-2299-2013, 2013.
Li, X.-D., Yang, Z., Fu, P., Yu, J., Lang, Y.-C., Liu, D., Ono, K., and Kawamura, K.: High abundances of dicarboxylic acids, oxocarboxylic acids, and α-dicarbonyls in fine aerosols (PM2.5) in Chengdu, China during wintertime haze pollution, Environ. Sci. Pollut. R., 22, 12902–12918, 2015.
Lim, H.-J., Carlton, A. G., and Turpin, B. J.: Isoprene forms secondary organic aerosol through cloud processing: Model simulations, Environ. Sci. Technol., 39, 4441–4446, 2005.
Lin, P., Hu, M., Deng, Z., Slanina, J., Han, S., Kondo, Y., Takegawa, N., Miyazaki, Y., Zhao, Y., and Sugimoto, N.: Seasonal and diurnal variations of organic carbon in PM2.5in Beijing and the estimation of secondary organic carbon, J. Geophys. Res., 114, D00G11, https://doi.org/10.1029/2008JD010902, 2009.
Liu, Y., Tao, S., Yang, Y., Dou, H., Yang, Y., and Coveney, R. M.: Inhalation exposure of traffic police officers to polycyclic aromatic hydrocarbons (PAHs) during the winter in Beijing, China, Sci. Total Environ., 383, 98–105, 2007.
Miyazaki, Y., Aggarwal, S. G., Singh, K., Gupta, P. K., and Kawamura, K.: Dicarboxylic acids and water-soluble organic carbon in aerosols in New Delhi, India, in winter: Characteristics and formation processes, J. Geophys. Res.-Atmos., 114, D19206, https://doi.org/10.1029/2009JD011790, 2009.
Miyazaki, Y., Kawamura, K., Jung, J., Furutani, H., and Uematsu, M.: Latitudinal distributions of organic nitrogen and organic carbon in marine aerosols over the western North Pacific, Atmos. Chem. Phys., 11, 3037–3049, https://doi.org/10.5194/acp-11-3037-2011, 2011.
Mkoma, S. L. and Kawamura, K.: Molecular composition of dicarboxylic acids, ketocarboxylic acids, α-dicarbonyls and fatty acids in atmospheric aerosols from Tanzania, East Africa during wet and dry seasons, Atmos. Chem. Phys., 13, 2235–2251, https://doi.org/10.5194/acp-13-2235-2013, 2013.
Mochida, M., Kawabata, A., Kawamura, K., Hatsushika, H., and Yamazaki, K.: Seasonal variation and origins of dicarboxylic acids in the marine atmosphere over the western North Pacific, J. Geophys. Res.-Atmos., 108, 4193, https://doi.org/10.1029/2002JD002355, 2003.
Pathak, R. K., Wang, T., Ho, K., and Lee, S.: Characteristics of summertime PM 2.5 organic and elemental carbon in four major Chinese cities: implications of high acidity for water-soluble organic carbon (WSOC), Atmos. Environ., 45, 318–325, 2011a.
Pathak, R. K., Wang, T., Ho, K. F., and Lee, S. C.: Characteristics of summertime PM2.5 organic and elemental carbon in four major Chinese cities: Implications of high acidity for water-soluble organic carbon (WSOC), Atmos. Environ., 45, 318–325, 2011b.
Pavuluri, C. M. and Kawamura, K.: Evidence for 13-carbon enrichment in oxalic acid via iron catalyzed photolysis in aqueous phase, Geophys. Res. Lett., 39, 3802, https://doi.org/10.1029/2011GL050398, 2012.
Pavuluri, C. M., Kawamura, K., and Swaminathan, T.: Water-soluble organic carbon, dicarboxylic acids, ketoacids, andα-dicarbonyls in the tropical Indian aerosols, J. Geophys. Res., 115, D11302, https://doi.org/10.1029/2009JD012661, 2010.
Pavuluri, C. M., Kawamura, K., Swaminathan, T., and Tachibana, E.: Stable carbon isotopic compositions of total carbon, dicarboxylic acids and glyoxylic acid in the tropical Indian aerosols: Implications for sources and photochemical processing of organic aerosols, J. Geophys. Res., 116, D18307, https://doi.org/10.1029/2011JD015617, 2011.
Ren, L. J., Fu, P. Q., He, Y., Hou, J. Z., Chen, J., Pavuluri, C. M., Sun, Y. L., and Wang, Z. F.: Molecular distributions and compound-specific stable carbon isotopic compositions of lipids in wintertime aerosols from Beijing, Sci. Rep.-UK, 6, https://doi.org/10.1038/srep27481, 2016.
Rogge, W. F., Hildemann, L. M., Mazurek, M. A., Cass, G. R., and Simoneit, B. R.: Sources of fine organic aerosol. 1. Charbroilers and meat cooking operations, Environ. Sci. Technol., 25, 1112–1125, 1991.
Rogge, W. F., Hildemann, L. M., Mazurek, M. A., Cass, G. R., and Simoneit, B. R.: Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks, Environ. Sci. Technol., 27, 636–651, 1993.
Rolph, G., Stein, A., and Stunder, B.: Real-time Environmental Applications and Display sYstem: READY, Environ. Modell. Softw., 95, 210–228, 2017.
Saxena, P., Hildemann, L. M., McMurry, P. H., and Seinfeld, J. H.: Organics alter hygroscopic behavior of atmospheric particles, J. Geophys. Res.-Atmos., 100, 18755–18770, 1995.
Schauer, J. J., Kleeman, M. J., Cass, G. R., and Simoneit, B. R.: Measurement of emissions from air pollution sources. 1. C1 through C29 organic compounds from meat charbroiling, Environ. Sci. Technol., 33, 1566–1577, 1999.
Schauer, J. J., Kleeman, M. J., Cass, G. R., and Simoneit, B. R.: Measurement of emissions from air pollution sources. 3. C1-C29 organic compounds from fireplace combustion of wood, Environ. Sci. Technol., 35, 1716–1728, 2001.
Simoneit, B. R., Medeiros, P. M., and Didyk, B. M.: Combustion products of plastics as indicators for refuse burning in the atmosphere, Environ. Sci. Technol., 39, 6961–6970, 2005.
Stephanou, E. G. and Stratigakis, N.: Oxocarboxylic and alpha, omega-dicarboxylic acids: photooxidation products of biogenic unsaturated fatty acids present in urban aerosols, Environ. Sci. Technol., 27, 1403–1407, 1993.
Sun, Y., Wang, Z., Dong, H., Yang, T., Li, J., Pan, X., Chen, P., and Jayne, J. T.: Characterization of summer organic and inorganic aerosols in Beijing, China with an Aerosol Chemical Speciation Monitor, Atmos. Environ., 51, 250–259, 2012.
Sun, Y., Du, W., Fu, P., Wang, Q., Li, J., Ge, X., Zhang, Q., Zhu, C., Ren, L., Xu, W., Zhao, J., Han, T., Worsnop, D. R., and Wang, Z.: Primary and secondary aerosols in Beijing in winter: sources, variations and processes, Atmos. Chem. Phys., 16, 8309–8329, https://doi.org/10.5194/acp-16-8309-2016, 2016.
Sun, Y. L., Wang, Z. F., Fu, P. Q., Yang, T., Jiang, Q., Dong, H. B., Li, J., and Jia, J. J.: Aerosol composition, sources and processes during wintertime in Beijing, China, Atmos. Chem. Phys., 13, 4577–4592, https://doi.org/10.5194/acp-13-4577-2013, 2013.
Sun, Y. L., Wang, Z. F., Du, W., Zhang, Q., Wang, Q. Q., Fu, P. Q., Pan, X. L., Li, J., Jayne, J., and Worsnop, D. R.: Long-term real-time measurements of aerosol particle composition in Beijing, China: seasonal variations, meteorological effects, and source analysis, Atmos. Chem. Phys., 15, 10149–10165, https://doi.org/10.5194/acp-15-10149-2015, 2015.
Sun, Z., Mu, Y., Liu, Y., and Shao, L.: A comparison study on airborne particles during haze days and non-haze days in Beijing, Sci. Total Environ., 456, 1–8, 2013.
Turekian, V. C., Macko, S. A., and Keene, W. C.: Concentrations, isotopic compositions, and sources of size-resolved, particulate organic carbon and oxalate in near-surface marine air at Bermuda during spring, J. Geophys. Res., 108, 347–362, 2003.
Turnhouse, M. B.: Role of 14C dating in paleontology, J. Paleontol., 75, 11–15, 1987.
Ulbrich, I. M., Canagaratna, M. R., Zhang, Q., Worsnop, D. R., and Jimenez, J. L.: Interpretation of organic components from Positive Matrix Factorization of aerosol mass spectrometric data, Atmos. Chem. Phys., 9, 2891–2918, https://doi.org/10.5194/acp-9-2891-2009, 2009.
Viana, M., López, J., Querol, X., Alastuey, A., García-Gacio, D., Blanco-Heras, G., López-Mahía, P., Piñeiro-Iglesias, M., Sanz, M., and Sanz, F.: Tracers and impact of open burning of rice straw residues on PM in Eastern Spain, Atmos. Environ., 42, 1941–1957, 2008.
Volkamer, R., Platt, U., and Wirtz, K.: Primary and secondary glyoxal formation from aromatics: Experimental evidence for the bicycloalkyl-radical pathway from benzene, toluene, and p-xylene, J. Phys. Chem. A, 105, 7865–7874, 2001.
Wang, G., Kawamura, K., Watanabe, T., Lee, S., Ho, K., and Cao, J.: High loadings and source strengths of organic aerosols in China, Geophys. Res. Lett., 33, 217–234, 2006.
Wang, G., Kawamura, K., Cheng, C., Li, J., Cao, J., Zhang, R., Zhang, T., Liu, S., and Zhao, Z.: Molecular distribution and stable carbon isotopic composition of dicarboxylic acids, ketocarboxylic acids, and α-dicarbonyls in size-resolved atmospheric particles from Xi'an City, China, Environ. Sci. Technol., 46, 4783–4791, 2012.
Wang, H. and Kawamura, K.: Stable carbon isotopic composition of low-molecular-weight dicarboxylic acids and ketoacids in remote marine aerosols, J. Geophys. Res.-Atmos., 111, D07304, https://doi.org/10.1029/2005JD006466, 2006.
Wang, H., Kawamura, K., and Yamazaki, K.: Water-soluble dicarboxylic acids, ketoacids and dicarbonyls in the atmospheric aerosols over the Southern Ocean and western Pacific Ocean, J. Atmos. Chem., 53, 43–61, 2006.
Warneck, P.: In-cloud chemistry opens pathway to the formation of oxalic acid in the marine atmosphere, Atmos. Environ., 37, 2423–2427, 2003.
Wu, S.-P., Schwab, J., Liu, B.-L., Li, T.-C., and Yuan, C.-S.: Seasonal variations and source identification of selected organic acids associated with PM 10 in the coastal area of Southeastern China, Atmos. Res., 155, 37–51, 2015.
Xia, X., Chen, H., and Zhang, W.: Analysis of the dependence of column-integrated aerosol properties on long-range transport of air masses in Beijing, Atmos. Environ., 41, 7739–7750, 2007.
Zhang, J. K., Sun, Y., Liu, Z. R., Ji, D. S., Hu, B., Liu, Q., and Wang, Y. S.: Characterization of submicron aerosols during a month of serious pollution in Beijing, 2013, Atmos. Chem. Phys., 14, 2887–2903, https://doi.org/10.5194/acp-14-2887-2014, 2014.
Zhang, Y. L., Kawamura, K., Cao, F., and Lee, M.: Stable carbon isotopic compositions of low-molecular weight dicarboxylic acids, oxocarboxylic acids, α-dicarbonyls, and fatty acids: Implications for atmospheric processing of organic aerosols, J. Geophys. Res.-Atmos., 121, 3707–3717, 2016.
Zhang, Y. Y., Müller, L., Winterhalter, R., Moortgat, G. K., Hoffmann, T., and Pöschl, U.: Seasonal cycle and temperature dependence of pinene oxidation products, dicarboxylic acids and nitrophenols in fine and coarse air particulate matter, Atmos. Chem. Phys., 10, 7859–7873, https://doi.org/10.5194/acp-10-7859-2010, 2010.
Zhao, P. S., Dong, F., He, D., Zhao, X. J., Zhang, X. L., Zhang, W. Z., Yao, Q., and Liu, H. Y.: Characteristics of concentrations and chemical compositions for PM2.5 in the region of Beijing, Tianjin, and Hebei, China, Atmos. Chem. Phys., 13, 4631–4644, https://doi.org/10.5194/acp-13-4631-2013, 2013.
Zhao, Y., Hu, M., Slanina, S., and Zhang, Y.: Chemical compositions of fine particulate organic matter emitted from Chinese cooking, Environ. Sci. Technol., 41, 99–105, 2007.
Zimmermann, J. and Poppe, D.: A supplement for the RADM2 chemical mechanism: the photooxidation of isoprene, Atmos. Environ., 30, 1255–1269, 1996.