the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
On the thermodynamic and kinetic aspects of immersion ice nucleation
Donifan Barahona
Heterogeneous ice nucleation initiated by particles immersed within droplets is likely the main pathway of ice formation in the atmosphere. Theoretical models commonly used to describe this process assume that it mimics ice formation from the vapor, neglecting interactions unique to the liquid phase. This work introduces a new approach that accounts for such interactions by linking the ability of particles to promote ice formation to the modification of the properties of water near the particle–liquid interface. It is shown that the same mechanism that lowers the thermodynamic barrier for ice nucleation also tends to decrease the mobility of water molecules, hence the ice–liquid interfacial flux. Heterogeneous ice nucleation in the liquid phase is thus determined by the competition between thermodynamic and kinetic constraints to the formation and propagation of ice. At the limit, ice nucleation may be mediated by kinetic factors instead of the nucleation work. This new ice nucleation regime is termed spinodal ice nucleation. The comparison of predicted nucleation rates against published data suggests that some materials of atmospheric relevance may nucleate ice in this regime.
- Article
(4185 KB) -
Supplement
(537 KB) - BibTeX
- EndNote
Ice nucleation in cloud droplets and aerosol particles leads to cloud formation and glaciation at low temperatures. It is often initiated by certain aerosol species known as ice nucleating particles (INPs) (Barahona et al., 2017; Cziczo et al., 2013; DeMott et al., 2003). These include dust, biological particles, metals, effloresced sulfate and sea salt, organic material and soot (Hoose and Möhler, 2012; Murray et al., 2012). Background INP concentrations may be influenced by aerosol emissions (Lohmann and Feichter, 2005), altering the formation and evolution of ice clouds and leading to an indirect effect on climate. The assessment of the role of INPs in climate is challenging due to the complexity of the atmospheric processes involving ice and the limited understanding of the ice nucleation mechanism of INPs (Myhre et al., 2013). Ice formation promoted by a particle completely immersed within the liquid phase, referred as “immersion freezing”, is likely the most common cloud glaciation pathway in the atmosphere (DeMott et al., 2003; Wiacek et al., 2010). Immersion freezing is involved in the initiation of precipitation and determines, to a large extent, the phase partitioning in convective clouds (Diehl and Wurzler, 2004; Lance et al., 2011; Murray et al., 2012; Wiacek et al., 2010).
The accurate representation of immersion ice nucleation is critical for the correct modeling of cloud processes in the atmosphere (Hoose and Möhler, 2012; Murray et al., 2012; Tan et al., 2016). Field campaign data have been used to develop empirical formulations relating the INP concentration to the cloud temperature, T, and saturation ratio, Si (Bigg, 1953; Fletcher, 1959; Meyers et al., 1992), and more recently to the ambient aerosol size and composition (DeMott et al., 2010; Niemand et al., 2012; Phillips et al., 2013). Empirical formulations provide a simple way to parameterize ice nucleation in atmospheric models (Barahona et al., 2014; Gettelman et al., 2012). However they may not be valid outside the conditions used in their development, particularly as different experimental techniques may result in a wide range of measured ice nucleation efficiencies (Hiranuma et al., 2015). Alternatively, the ice nucleation efficiency can be empirically parameterized using laboratory data, although with similar caveats (Knopf and Alpert, 2013; Murray et al., 2012).
Molecular Dynamics (MD) simulations and direct kinetic methods have been used to study ice nucleation (Espinosa et al., 2014; Lupi et al., 2014; Matsumoto et al., 2002; Taylor and Hale, 1993). However, the classical nucleation theory (CNT) is nearly the only theoretical approach employed to describe immersion freezing in cloud models (Barahona and Nenes, 2008, 2009; Hoose et al., 2010; Khvorostyanov and Curry, 2004). According to CNT, nucleation is initiated by the growth of a cap-shaped ice germ on the surface of the immersed particle (Kashchiev, 2000; Pruppacher and Klett, 1997). The geometry of the ice germ is defined by a force balance at the particle–ice–liquid interface and is characterized by the contact angle, θ. In this sense, the ice germ is assumed to “wet” the immersed particle in the same way a liquid droplet wets a solid surface (De Gennes, 1985). Low values of θ indicate a high affinity of the particle for ice and a low energy of formation of the ice germ.
Direct application of CNT in immersion freezing is thwarted by uncertainty in fundamental parameters of the theory, i.e., the ice–liquid interfacial energy, σiw, and the activation energy. Moreover, using a single θ to describe the nucleation efficiency of dust and other materials typically leads to a large discrepancy between CNT predictions and experimental measurements (Alpert et al., 2011; Broadley et al., 2012; Rigg et al., 2013; Zobrist et al., 2007). MD simulations show that an ice germ formed near a surface tends to have a complex geometry instead of the cap-shaped assumption of CNT (Cox et al., 2015; Fitzner et al., 2015; Lupi et al., 2014). Within a liquid the ice germ may not “wet” the particle but may rather exert stress on the substrate (Cahn, 1980; Rusanov, 2005), and it is not clear that this can be described as a simple function of θ (Cahn, 1980). It has also been shown that σiw obtained by fitting CNT to measured nucleation rates tend to be biased to account for the mixing effects neglected in common formulations of the theory (Barahona, 2014).
More fundamentally, CNT neglects important interactions near the immersed particle that may influence the nucleation rate. It is assumed that ice nucleation solely depends on the local geometry of the absorbed molecules on the immersed particle (Kashchiev, 2000). This implies that the particle influences the formation of the ice germ but does not influence its adjacent water. The viscosity and density of water in the vicinity of the particle and in contact with the ice germ are assumed similar to those in the bulk (Kashchiev, 2000; Pruppacher and Klett, 1997). This is at odds with evidence of a strong effect of immersed particles on the vicinal water (Drost-Hansen, 1969; Michot et al., 2002). In fact, such an effect may be responsible for the enhancement of ice nucleation near immersed solids (Anderson, 1967).
1.1 Evidence for the formation of ordered structures near the liquid–particle interface
It has been known for some time that water near interfaces displays physicochemical properties different from those of the bulk (Bellissent-Funel, 2002; Drost-Hansen, 1969; Michot et al., 2002). By examining a wealth of reported experimental observations, Drost-Hansen (1969) concluded that vicinal water (i.e., the water immediately adjacent to the particle) may exist in an ordered state near the solid–liquid interface that may propagate over considerable distance, of the order of hundreds to thousands of molecular diameters. More recent experiments showing that hydrophilic surfaces have a long-range impact further support this conclusion (Zheng et al., 2006). The interaction between the particle and the vicinal water becomes more significant as the temperature decreases and the viscosity increases (Wolfe et al., 2002). Recent studies have shown the presence of ordered water near the interface of biological (Cooke and Kuntz, 1974; Snyder et al., 2014), metallic (Michot et al., 2002) and clay (Rinnert et al., 2005; Yu et al., 2001) particles, a notion that is also supported by molecular dynamics simulations (Cox et al., 2015; Lupi et al., 2014). In a groundbreaking work, Anderson (1967) found strong evidence of ice formation several molecular diameters away from the clay–water interface. The author concluded that ice formation does not require an ice germ attached to the substrate, but rather the nascent ice germ is stabilized by ordering in the interfacial zone. To date no quantitative theory has been developed exploiting such a view of ice nucleation.
The description of the properties of the vicinal water is still under investigation. In the supercooled region the presence of structured low-density water near solid surfaces (termed “ice-like”) has been reported for different materials (Etzler, 1983; Feibelman, 2010; Michaelides and Morgenstern, 2007; Snyder et al., 2014; Yu et al., 2001). In this region Etzler (1983) parameterized the density and enthalpy of vicinal water as a mixture of ice-like and bulk-like water. Additional experimental observations also show the modification of the mobility of water near interfaces and a higher viscosity than the bulk (Wang et al., 2006; Warne et al., 2000; Wolfe et al., 2002; Yu et al., 2001). In some cases, clays and biological systems exhibit a viscous layer of water at the particle–liquid interface that remains liquid even if the bulk has already frozen (Drost-Hansen, 1969). These effects are typically characterized as non-equilibrium, since they affect the flux of molecules to the nascent ice germ rather than the thermodynamics of ice nucleation. Li et al. (2014) found experimentally that the viscosity of interfacial water regulates the ice nucleation activity, giving support to the idea that the work of nucleation and the enhancement of the viscosity in the interfacial region are tightly linked. In fact, increased viscosity may be a necessary condition for ice nucleation, since structural ordering is not possible in a fluid with low viscosity (Anderson, 1967).
These considerations are largely missing in the theoretical description of ice nucleation. There is currently no theory that can account for the thermodynamic and kinetic effects of an immersed particle on the surrounding water, hence on ice nucleation. Such a task is undertaken in this work. Section 2 presents the theoretical description of a new approach, accounting for the thermodynamics of vicinal water (Sect. 2.3) and its relation to the work of nucleation (Sect. 2.4) and the nucleation rate (Sects. 2.5 and 2.6). These new relations are analyzed and applied to specific cases of atmospheric relevance in Sect. 3.
The new approach is developed within the scope of the kinetic treatment of nucleation, when cluster formation is the limiting step to ice formation (Kashchiev, 2000; Pruppacher and Klett, 1997). The central result of this theory is the following well-known general expression for the nucleation rate in steady state (Kashchiev, 2000):
where Z is the Zeldovich factor, f* is the attachment frequency (also called the impingement factor), and C* is the concentration of supercritical clusters. Z corrects for the detachment of monomers from the cluster during nucleation. It can also be interpreted as the probability that a molecule reaches the ice germ following a thermally activated “random walk”. Generally,
where ΔG is the work of cluster formation and n* is the number of water molecules in the ice germ. If the molecular cluster size distribution can be assumed to be near equilibrium, which is the case for immersion freezing, then
where ΔG* is the work of critical germ formation and C0 is the monomer concentration adjacent to the surface of the growing ice germ, implying that interface transfer is the dominant mechanism of cluster growth.
These expressions can be applied directly to model ice nucleation as follows. For homogeneous ice nucleation, and , with vw being the molecular volume of water (Pruppacher and Klett, 1997):
For heterogeneous ice nucleation, and , with being a0, the average cross-sectional area of a water molecule, i.e.,
Using is advantageous, because Jhet is typically normalized to the particle surface area (Hoose and Möhler, 2012; Murray et al., 2012). It, however, involves the assumption that the density of water does not vary within the droplet, remaining constant even at the particle–water interface. In other words, anywhere within the liquid, the per-area molecular density should be the same. This assumption, however, does not lead to significant error, since the effect of the particle on the water density is small (Etzler, 1983), and Jhet is linearly related to C0.
Equation (5) provides the basis for this work. It shows that, to predict the effect of the immersed particle on ice formation, it is necessary to understand how the presence of the particle affects ΔGhet and . To accomplish this Sect. 2.1 provides an overview of the main assumptions of CNT, which are then contrasted with the negentropic nucleation framework (NNF) in Sect. 2.2. The latter is used in Sects. 2.3 and 2.4 to analyze the thermodynamic aspects of immersion ice nucleation and to formulate a new expression for ΔGhet. Section 2.5 develops an expression for accounting for the effect of the particle on the mobility of water molecules. In Sect. 2.6 a new expression for the nucleation rate is formulated.
2.1 Classical nucleation theory
Since CNT is the most widely used theoretical approach in atmospheric models we start by highlighting its main characteristic. Common CNT expressions use several assumptions to simplify the description of the interaction between water and the immersed particle (Hoose et al., 2010; Khvorostyanov and Curry, 2004; Zobrist et al., 2007). Typically the particle is assumed to have a negligible effect on the mobility and the thermodynamics of the vicinal water, i.e., . The latter is calculated assuming that the formation of clusters within the liquid phase mimics a first-order reaction in an ideal gas where every molecule that randomly jumps into the ice–liquid interface is incorporated within the ice lattice. Thus is the product of the frequency factor (derived from transition state theory) and the monomer concentration at the ice–liquid interface. This leads to (Kashchiev, 2000; Pruppacher and Klett, 1997)
where ΔGact is the activation energy, i.e., the energy required for a water molecule to leave its equilibrium position in the bulk towards the vicinity of the ice germ (Pruppacher and Klett, 1997; Zobrist et al., 2007), h is Plank's constant, Ω the surface area of the ice germ and d0 is the molecular diameter of water.
The work of ice nucleation results from the assumption that the ice germ has a hemispherical shape. Other assumptions include no surface stress (Cahn, 1980) and negligible mixing effects during germ formation (Barahona, 2014). These considerations lead to the expression (Turnbull and Fisher, 1949)
where ΔGhom,CNT is the homogeneous work of nucleation, given by
where σiw is the ice–water interfacial energy and Si is the saturation ratio with respect to ice. The effect of the immersed particle on Jhet,CNT depends on the adsorption of water molecules on individual sites, and is characterized by the contact angle, θ, in the form
Equation (9) can be extended to account for line tension, curvature and misfit effects (Khvorostyanov and Curry, 2004), which, however, requires the usage of additional unconstrained parameters. Introducing Eqs. (6) and (7) into Eq. (5) we obtain the known expression,
where , and is the radius of the ice germ. Other symbols are defined in Table 1.
Due in part to the assumption of a negligible effect of the particle on the adjacent water the CNT framework does not provide a way to link the properties of the vicinal water to the nucleation rate. Another caveat of CNT is that fundamental parameters like ΔGact, σiw and θ do not have a clear definition outside of the context of the theory. For example, ΔGact is typically assumed the same as in bulk water, representing a barrier to bulk diffusion instead of interfacial transfer (Barahona, 2015; Kashchiev, 2000). Similarly σiw is not well defined for a diffuse interface, and it is difficult to measure away from equilibrium. Moreover, θ relies on a droplet-like picture of the nascent ice germ, which may not be appropriate for a germ forming within the dense liquid phase (Brukhno et al., 2008). Most studies thus treat ΔGact, σiw and θ as empirical parameters, fitted to match measured nucleation rates. Many times this results in complex functional forms of T and Si that may not be easily expanded to account for the modified properties of water near the immersed particle.
2.2 Negentropic nucleation framework
Some of the caveats of CNT are addressed in the negentropic nucleation framework (Barahona, 2014, 2015). In NNF simple thermodynamic arguments are used to approximate ΔGhom and fhom in terms of water properties that could, in principle, be independently estimated. This obviates the need for parameters that should be fitted to measured nucleation rates. At the same time, NNF is a relatively simple framework that can be easily implemented in large-scale atmospheric models and that has been shown to reproduce homogeneous freezing temperatures down to 180 K (Barahona, 2015; O and Wood, 2016). This section presents the main results of NNF for homogeneous ice nucleation.
In NNF the energy of formation of the interface, Φs, is an explicit function of the water activity and temperature in the form
where the constants Γw=1.46 and define the coverage of the ice–water interface and the lattice geometry of the ice germ, respectively, and Δhf is the latent heat of fusion of water. Other symbols are defined in Appendix A. Equation (11) results from accounting for the finite character of the ice–liquid interface and from the assumption that, in joining the ice lattice, the water molecules lose most of their entropy (Barahona, 2014). The driving force for ice nucleation, Δμi, is given by
where aw,eq is the equilibrium water activity. Equation (12) accounts for the work of “unmixing” affecting the bulk of the liquid when the ice germ is formed, which is proportional to ln(aw) (Black, 2007). Using Eqs. (11) and (12), the critical germ size and the work of nucleation are obtained from the condition of mechanical equilibrium of the ice germ (Barahona, 2014), resulting in
and
In more recent work the kinetics of homogeneous ice nucleation have been re-examined in NNF to account for molecular rearrangement during the transfer of water molecules across the ice–liquid interface (Barahona, 2015). Within this approach is determined by the diffusion coefficient for interfacial transfer, D, in the form (Barahona, 2015; Kashchiev, 2000)
where Ω is the surface area of the ice germ. D represents contributions from purely diffusive process and from structural transformations required to incorporate water molecules into the ice germ. The latter originates because neighboring molecules need to be rearranged to accommodate new ones into the ice lattice, generating entropy and dissipating work. Using considerations from non-equilibrium thermodynamics D can be written in the form (Barahona, 2015)
where D∞ is the bulk self-diffusion coefficient of water, and Wd is the average dissipated work during interface transfer. The latter is proportional to the excess free energy of solidification of water, i.e., , with nt=16, the number of possible trajectories in which individual water molecules can make four-bonded water. Equation (16) shows explicitly that bulk diffusion (i.e., D∞) as well as structural rearrangement are required for ice germ growth. Introducing Eq. (16) into Eq. (15) we obtain
Application of Eq. (17) to homogeneous ice nucleation shows agreement of Jhom with experimental data at very low T, where kinetic processes dominate the formation ice (Barahona, 2015).
NNF provides explicit dependencies of D and Φs on thermodynamic properties without depending on nucleation rate measurements. Thus it provides a suitable basis to study the thermodynamics and kinetics of ice formation in the vicinity of immersed particles. Doing so first requires building a model to describe the thermodynamics of the vicinal water.
2.3 Thermodynamics of the liquid–particle interface
The discussion presented in Sect. 1.1 suggests that the immersed particle enhances order near the particle–liquid interface, lowering the energy required to nucleate ice. The vicinal layer is thus described as a solution of hypothetical ice-like (IL) and liquid-like (LL) regions, with Gibbs free energy, given by
where and are the chemical potentials of the LL and IL species within the solution, respectively, and ζ is the fraction of IL regions in the layer. Increased order is represented by a higher fraction of IL regions, hence higher ζ. Equation (18) can also be written in terms of the chemical potentials of the “pure” LL and IL species, μLL and μIL, respectively, in the form
where is the Gibbs energy of mixing. For a mechanical mixture of pure LL and IL species, ΔGmix=0, whereas for an ideal solution, ΔGmix is determined by the ideal entropy of mixing (Prausnitz et al., 1998). Reorganizing Eq. (19) we obtain,
where . Δμil can be approximated using the equilibrium between bulk liquid and ice as a reference state (Kashchiev, 2000), making
and
where aw,eff is termed the “effective water activity” and is the value of aw associated with the LL regions in the vicinal water, and aIL is the water activity in the IL regions. Assuming that, similarly to bulk ice, the solute does not significantly partition to the IL phase, then aIL≈1. With this, and by combining Eq. (21) and Eq. (22) and rearranging, we obtain
The central assumption behind Eq. (23) is that aw,eq corresponds to the equilibrium water activity between liquid and ice, or in other words, that near equilibrium Δμil≈Δμs. In reality Δμs corresponds to actual liquid and ice, instead of the hypothetical LL and IL substances. This difference can be accounted for by selecting a proper functional form for ΔGmix, for which several empirical and semi-empirical interaction models, with varying degrees of complexity, exist (Prausnitz et al., 1998). In this work it is assumed that the vicinal water can be described as a regular solution. This is the simplest model that accounts for the interaction between solvent and solute during mixing and that is flexible enough to include corrections for the difference between Δμs and Δμil. Using this model Holten et al. (2013) were able to approximate the chemical potential of supercooled water. The authors also showed that taking into account clustering of water molecules led to a better agreement of the estimated water properties with MD simulations and experimental results.
According to the regular solution model, modified by clustering Holten et al. (2013),
The first term on the right-hand side corresponds to the usual definition of the ideal entropy of mixing, i.e., random ideal mixing and weak interaction between IL and LL regions, modified to account for clustering in groups of N molecules. N=6 corresponds to clustering in hexamers and is near the optimum fit between MD simulations and the solution model (Holten et al., 2013). It must be noted that Holten et al. (2013) recommended an alternative model termed “athermal solution”, where nonideality is ascribed to entropy changes upon mixing. In vicinal water some evidence points to nonideality originating from enthalpy changes near the particle (Etzler, 1983); hence a regular solution is more appropriate in this case. For N=6 the difference between the two models is negligible (Holten et al., 2013).
The second term on the right-hand side of Eq. (24) is an empirical functional form used to approximate the enthalpy of mixing, selected so that ΔGmix=0 for both ζ=0 and ζ=1. Aw is a phenomenological interaction parameter, here assumed to implicitly correct the approximation Δμil≈Δμs. Typically Aw must be fitted to experimental observations. In this work Aw is calculated using an alternative approach, as follows.
An important aspect of the regular solution model is that it predicts that μvc has a critical temperature, Tc, defined by the conditions,
These conditions originate because represents a stability limit for the vicinal water. A solution would split into two phases, if doing so lowers its Gibbs free energy (Prausnitz et al., 1998). For a metastable solution μvc must be minimal, hence . The condition indicates that any increase in ζ increases μvc (i.e., the curve μvc vs. ζ becomes concave downward), such that it is thermodynamically more favorable for the solution to split into distinct phases than to increase its concentration. The last condition, , indicates that the metastable region reduces to a single point. Using Eq. (20) into Eq. (25) we obtain,
and
The last expression is only valid for ζ=0.5, indicating that a single critical temperature exists for a regular solution. Using this in Eq. (26) and solving for Aw gives, for T=Tc,
Physically, Tc represents the stability limit of the vicinal water, at which it spontaneously separates into IL and LL regions. For T<Tc the chemical potential of an equimolar solution of IL and LL would be larger than that of a simple mechanical mixture of the two species. Thus it is thermodynamically more favorable for the solution to split into its individual components, i.e., ice and liquid, leading to a stability limit of the system. Equation (28) thus provides an opportunity to theoretically determine Aw, since Tc should also correspond to a negligible work of nucleation. This further explained in Sect. 2.4.2.
Introducing Eqs. (23), (24) and (28) in Eq. (20), we obtain
By defining Λmix in the form,
Equation (29) can be written in the form
Equation (31) is the equation of state of the vicinal water. It describes the properties of the vicinal water in terms of the material-specific parameter ζ and the interaction parameters N and Tc. MD simulations indicate that N∼6 (Bullock and Molinero, 2013; Holten et al., 2013). Tc is thus the only remaining unknown in Eq. (31) and is calculated in Sect. 2.4.2.
2.4 Work of germ formation
The equation of state of vicinal water can be used to link ΔGhom and ΔGhet as follows. In immersion freezing the particle remains within the droplet long enough that equilibrium is established. This condition is mathematically expressed by the equality, μvc=μw, where μw is the chemical potential of water in the bulk of the liquid, i.e., away from the particle. Using Eq. (31) this implies that
This expression indicates that the effect of the particle on its vicinal water can be understood as an enhancement of the chemical potential of the LL regions, a consequence of the tendency of the particle to lower μvc. Since Δμil<0, μLL must increase to maintain equilibrium. Using the equilibrium between bulk liquid and ice as reference state so that , we obtain the following after simplifying:
Or, equivalently,
Equation (34) suggests that, thermodynamically, immersion freezing can be described as homogeneous ice nucleation occurring at an enhanced water activity. This is because it is possible to create a path including homogeneous ice nucleation with the same change in Gibbs free energy as for heterogeneous freezing. Figure 1 shows that for a particle-droplet system in equilibrium, aw,eff satisfies the condition
Equation (35) represents a thermodynamic relation between ΔGhet and ΔGhom. It has the advantage that ΔGhet can be obtained without invoking assumptions on the mechanistic details of the interaction between the particle and the ice germ, which are parameterized by ζ. Since aw is typically the controlled variable in ice nucleation, aw,eff can be readily obtained by solving Eq. (34),
Although ascribing ice nucleation to the LL fraction of vicinal water agrees with the decisive role of free water in the formation of ice (Wang et al., 2016), caution must be taken in considering this to be the actual mechanism of ice nucleation, which could be quite complex. Equation (35), however, establishes a thermodynamic constrain for ΔGhet that should be met by any ice nucleation mechanism. It is also important to analyze the behavior of aw,eff as ζ→1. It can be shown that the quotient converges for ζ→1 as follows. From Eq. (30) we can write
Using for x→1, the last expression can be shown to converge to
The fact that for T≠Tc stems from the simple interaction model used to define ΔGmix (i.e., the regular solution approximation). Tc may depend on ζ, however the regular solution approximation predicts a unique critical temperature at ζ=0.5. This, however, does not lead to uncertainty in ΔGhom since for ζ→1, the first term on the right-hand side of Eq. (36) is almost singular at . Thus if , then aw must be just above aw,eq to make . In other words, for all practical purposes, when the system approaches thermodynamic equilibrium.
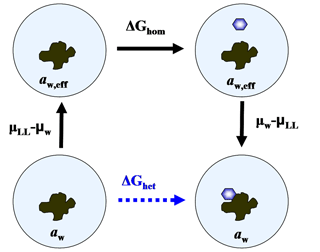
Figure 1Diagram representing a thermodynamic path, including homogeneous ice nucleation with the same work as heterogeneous freezing.
2.4.1 Extension of the homogeneous model to the spinodal limit
In applying the homogeneous model to the heterogeneous problem in the form detailed in Sect. 2.4, caution must be taken in describing the limiting condition where the size of the ice germ becomes exceedingly small, i.e., nhom→1, representing the vanishing of the energy barrier to ice nucleation. This is possible, since as ζ→1, aw,eff becomes large (Eq. 36), and for ζ=1 it is only defined at thermodynamic equilibrium. Since for nhom→1, thermodynamic potentials are not well defined, it is necessary to test the validity of NNF at such a limit. Moreover, in its original formulation (Sect. 2.2) NNF predicts a positive ΔGhom for nhom=1, at odds with the notion that the formation of a monomer-sized germ should carry no work.
At the limiting condition, nhom=1, the work of nucleation is smaller than the thermal energy of the molecules and represents the onset of spontaneous phase separation (termed “spinodal decomposition”) during nucleation (Vekilov, 2010). Here it is argued that being a far-from-equilibrium process, ice nucleation always carries energy dissipation. When accounted for, the apparent inconsistency in NNF at nhom=1 vanishes, since as shown below such a condition is not accessible. This approach differs from previous treatments, where this limit is associated with a negligible driving force for nucleation (Kalikmanov and van Dongen, 1993).
To account for the finite, albeit small, amount of work dissipated from the generation of entropy during spontaneous fluctuation, a simple approach is proposed. It involves writing the work of cluster formation in the form
where Wdiss represents work dissipation, assumed independent of the germ size, since it results from spontaneous fluctuations occurring in the liquid phase. Equation (39) is the typical expression for ΔG (Barahona, 2014) with an additional term accounting for irreversibility. The nucleation work is defined for n=nhom in the form
where nhom is obtained from the mechanical stability condition, , and is still given by Eq. (13), since Wdiss is assumed independent of n. Wdiss is then obtained from the conditions
The first condition expresses the fact that the formation of a monomer-sized ice germ carries no work. The second condition establishes that nhom=1 should correspond to a stability limit of the system where nucleation and spontaneous separation are analogous. This is referred as the spinodal point. From Eq. (40) we obtain
Since nhom only attains positive values, then only the trivial solution Φs=0 satisfies Eq. (42), i.e., the energy barrier to the formation of the ice germ vanishes at the spinodal. Using Φs=0 and , Eq. (40) can be solved for Wdiss in the form
Thus the minimum amount of work dissipated during nucleation corresponds to a fluctuation relaxing Δμi. Replacing this expression within Eq. (40), we obtain
Using Eq. (13) in Eq. (44) gives, after rearranging, the work of germ formation by homogeneous ice nucleation:
Equation (45) only differs from the NNF expression, Eq. (14), on the right-hand side, where it is implied that nucleation in a solution requires the coordination of at least two molecules, a condition that has been observed experimentally in the crystallization of proteins (Vekilov, 2010). It also suggests that dissipation effects are negligible for typical homogeneous nucleation conditions, i.e., , since nhom∼200 (Barahona, 2014). Moreover, the fact that ΔGhom>0 even when nhom→0 implies that ice nucleation always requires some work. Using Eq. (35) the heterogeneous work of nucleation can be readily written as
Equation (46) also suggests an operational definition for the critical ice germ in immersion freezing in the form
The results of Eqs. (45) and (46) require further explanation, since in principle, an ice germ with only two molecules does not exist. Thus Eq. (45) must be interpreted in a different way. As ζ→1, or in deeply supercooled conditions, the fraction of ice-like regions in the vicinal water becomes large. Under such a scenario the reorientation of only two molecules may be enough to initiate ice nucleation. In other words, beyond the spinodal point ice nucleation is controlled by molecular motion within already formed ice-like regions. For homogeneous ice nucleation this would require extreme supercooling (T∼140 K, Fig. 2). In immersion ice nucleation it may occur at higher T, since the formation of a high fraction of ice-like regions in the vicinal water is facilitated by efficient INPs. This is further explored in Sect. 3.
NNF carries the assumption that thermodynamic potentials can be defined for the ice germ. In other words nhom should be large enough that it represents a statistical ensemble of molecules. Of course this is not the case for nhom=1, and it may cast doubt on the application of Eq. (39) to such limits. This possibility is, however, mitigated in two ways. Unlike CNT, which is based on the interfacial tension, the NNF framework is robust for small germs. Size effects impact ΔG mostly through Φs, since Δμi does not change substantially with the size of the system. In NNF the product ΓwsΔhf in Eq. (11) remains constant, and Φs is relatively insensitive to n. This is because Δhf decreases with n as the total cohesive energy of the germ is inversely proportional to the number of molecules within the ice–liquid interfacial layer (Johnston and Molinero, 2012; Zhang et al., 1999). At the same time, the product Γws, i.e., the ratio of the number of surface to interior molecules in the germ (Barahona, 2014; Spaepen, 1975), should increase for small ice germs, offsetting the decrease in Δhf. Such behavior is supported by MD simulations (Johnston and Molinero, 2012). Equation (11) thus remains valid for small germs. A second mitigating factor is discussed in Sect. 3.1, where it is shown that conditions leading to nhet→1 are rare in the atmosphere, and Jhet is largely independent of nhet for very small germs.
2.4.2 Critical temperature
To complete the thermodynamic description of ice nucleation near the particle–liquid interface it is necessary to specify the critical separation temperature defined in Eq. (28). The criterion used to find Tc is that the reversible work of nucleation, that is, without accounting for the dissipation term, becomes negligible. Wdiss is not included, since the definition of ΔGmix (Eq. 24), does not account for such effects.
An analysis of Eqs. (44) and (46) reveals that ΔGhom (hence ΔGhet) is at a minimum when the reversible work becomes negligible. As T decreases, Δμi increases, decreasing nhom and ΔGhom. However, as nhom→0, the tendency is reversed, since ΔGhom∼Wdiss. In this regime dissipative effects dominate, and ΔGhom and ΔGhet become proportional to Δμi (Fig. 2). Thus the minimum in ΔGhet signals nhom→0 and nhet→2. If no dissipation or kinetic effects were present (for example at low supercooling), then phase separation would ensue, since the work of nucleation would be smaller than the thermal energy of the water molecules. This limit should also correspond to the stability limit of the vicinal water where IL and LL species separate spontaneously; hence it can be used to find Tc. It must be noted that this criterion does not mean that ΔGhet=0 at T=Tc but rather that such would be the case for a thermodynamically reversible nucleation process.
In the regular solution model the interaction parameter Aw is defined for Tc at ζ=0.5 (Sect. 2.3). Thus to find Tc we look for the temperature that would produce a minimum in ΔGhet at ζ=0.5. Mathematically, this is the temperature that simultaneously satisfies the conditions described in Eqs. (25) and (41). Figure 2 shows that this occurs around T∼211 K for ζ=0.5. Since both ΔGhet and aw,eff depend on Tc, we can iteratively solve Eqs. (36) and (46) to find Tc=211.473 K. Figure 2 also suggests that when T remains constant there is a critical value of ζ that marks the transition between two thermodynamic regimes. This is analyzed in Sect. 3.1.
2.5 Kinetics of immersion freezing
Almost every theoretical approach to describe the effect of INPs on ice formation focuses on the thermodynamics of ice nucleation. However as discussed in Sect. 1.1, increased molecular ordering increases the viscosity of vicinal water, implying that the immersed particle modifies the flux of water molecules to the nascent ice germ, hence the kinetics of ice nucleation (Etzler, 1983; Feibelman, 2010). Since these structural changes are also related to modifications in the chemical potential of the vicinal water, it is likely that the same mechanism that decreases ΔGhet also controls the mobility of water molecules in the environment around the particle. Such a connection between the water thermodynamic properties and its molecular mobility is well established (Adam and Gibbs, 1965; Debenedetti and Stillinger, 2001; Scala et al., 2000), but it is generally neglected in nucleation theory (Ickes et al., 2017; Pruppacher and Klett, 1997). In this section a heuristic model is proposed to account for such effects.
Kinetic effects modify the value of the impingement factor, , which controls the flux of water molecules to the ice germ. In general the ice germ grows by diffusion and rearrangement of nearby water molecules across the ice–liquid interface, characterized by the interfacial diffusion coefficient, D. Increased ordering is characterized by a higher IL fraction, hence higher ζ. Thus, in immersion freezing, D must be a function of ζ. Using Eq. (15) this can be expressed in the form
Assuming that within the vicinal layer the ice germ grows following a similar mechanism as in the bulk of the liquid, then Eq. (16) can be applied to the heterogeneous process in the form
The last expression indicates that ice–liquid interfacial transfer requires a diffusional and a rearrangement component. D∞(ζ) characterizes purely diffusional processes occurring within the particle–liquid interface. Molecular rearrangement during ice germ growth within the vicinal layer is determined by Wd(ζ). Since only molecules in the LL fraction of the vicinal water would rearrange to join the ice lattice then the latter is given by
Introducing the last expression in Eq. (49) we obtain
This expression is consistent with the thermodynamic model presented in Sect. 2.3, since as ζ increases, the vicinal water has a larger “ice” character, and fewer molecules need to rearrange to be incorporated into the growing ice germ.
2.5.1 Diffusion within the particle–liquid interface
The diffusional component of D corresponds to the random jump of water molecules across the ice–liquid interface. For ζ→0 there is no interaction between the particle and the adjacent water, hence diffusion must proceed as in the bulk of the supercooled water. At the opposite limit, ζ→1 and D∞(ζ)→0, which simply states that interfacial transfer vanishes when no net driving force exists across the ice–liquid interface, i.e., the system is in equilibrium. To model this behavior the well-known relaxation theory proposed by Adam and Gibbs (1965) is employed (hereinafter, AG65). According to AG65, relaxation and diffusion in supercooled liquids require the formation of cooperative regions (CRs). The average transition probability and the timescale of diffusion are determined by the size of the smallest CR. Following a statistical mechanics treatment and assuming that each CR interacts weakly with the rest of the system, the authors derived the following expression for the average transition probability:
where A represents the product of the minimum size of a CR in the liquid and the energy required to displace water molecules from their equilibrium position in the bulk, and Sc is the configurational entropy. Since A is approximately constant, the mobility of water molecules is controlled by Sc, which has been confirmed in molecular dynamics simulations and experimental studies (Debenedetti and Stillinger, 2001; Scala et al., 2000). The self-diffusivity of water is proportional to the transition probability and can be expressed in the form , where D0 is a constant. Using Eq. (52) this suggests the relationship
where and represent values in the bulk of the liquid. Equation (53) implies that the flux of molecules to the ice germ during immersion freezing is controlled by the configurational entropy of vicinal water. The usage of Eq. (53) thus requires developing an expression for Sc, which is approximated in the form
where Sc,LL and Sc,IL are the configurational entropies of the LL and IL fractions, respectively. The term Sc,LL in Eq. (54) dominates Sc, since diffusion is controlled by molecules mobile enough to be incorporated in CRs (Stanley and Teixeira, 1980), although Sc,IL determines Sc when ζ→1.
The regular model proposed in Sect. 2.3 suggests a weak interaction between IL and LL regions, since ΔGmix is small compared to μvc. Thus we can approximate that . Unfortunately equating Sc,IL to the configurational entropy of bulk ice (which can be deduced from geometrical arguments; Pauling, 1935) would violate the requirement that D→0 at thermodynamic equilibrium. To estimate Sc,IL we assume instead that water molecules in the IL regions should be displaced from their equilibrium position (essentially “diffusing” into the LL regions) to be incorporated into the ice lattice. During this process they gain an amount of energy equal to −Δμs which is returned to the system upon entering the ice–liquid interface. Since this energy exchange results mostly from configurational rearrangement we can approximate that (Barahona, 2014; Spaepen, 1975). With this, and using , Eq. (54) can be rewritten in the form
By introducing this expression into Eq. (53) and rearranging, we obtain
where . Using , an equivalent expression to Eq. (56) can be written in the form
Equation (57) represents the effect of the immersed particle on the rate of growth of the ice germ. For ζ=0, the particle does not affect the flux of water molecules to the nascent ice germ and D∞(ζ)=D∞. However as ζ→1, , and interface transfer becomes severely limited, particularly near equilibrium, since σE→1. This effect is much stronger than the reduction in the dissipated work from an increased ζ (Sect. 2.5) and dominates D.
Introducing Eqs. (51) and (57) into Eq. (48) and rearranging, we obtain
where was used.
2.6 Nucleation rate
The results of Sects. 2.3 to 2.5 provide the basis for writing an expression for the ice nucleation rate of droplets by immersion freezing. Before completing such a description we need to provide an expression for Z. The application of Eq. (2) typically leads to the known expression (Pruppacher and Klett, 1997)
On the other hand using Eq. (46) in Eq. (2), we obtain
where the subscript “d” indicates that energy dissipation is taken into account. For nhet>3 it is easily verifiable that Zd≈Z. Indeed the discrepancy between Zd and Z is only 30 % for nhet=3, and it is much smaller for larger ice germs. However for nhet=2, Zd=0. This issue is rather fundamental and may represent the breaking of the assumption that each germ grows by the addition of a single molecule at a time. Hence Eq. (59) will be used keeping in mind that for very small ice germs, it represents only an approximation.
With the above considerations it is now possible to substitute Eqs. (46), (47), (58) and (59) into Eq. (5) to obtain the heterogeneous ice nucleation rate
where and were used, and is the surface area of the ice germ. Other symbols and values used are listed in Appendix A.
2.7 The role of active sites
There is evidence that in dust and other INPs, ice is formed preferentially in the vicinity of surface patches, commonly referred as active sites. The existence of active sites has been established experimentally for deposition ice nucleation Kiselev et al. (2017), and they may be also important for immersion freezing Murray et al. (2012). In the classical view active sites have the property of locally reducing ΔGhet, increasing Jhet. In the so-called singular hypothesis each active site has an associated characteristic temperature at which it nucleates ice. Current interpretation assigns Jhet→∞ at each active site at its characteristic temperature, with some variability due to “statistical fluctuations” in the germ size (Vali, 2014). Some CNT-based approaches to describe immersion freezing account for the existence of active sites by assuming a distribution of contact angles for each particle. Hence each active site is assigned a characteristic contact angle instead of a characteristic temperature (Ickes et al., 2017; Zobrist et al., 2007).
The view of the role of active sites as capable of locally decreasing ΔGhet relies heavily on an interpretation of immersion freezing that mimics ice nucleation from the vapor phase (Fig. 3a). Such a description is, however, too limited for ice formation within the liquid phase. For example, it is implicitly assumed that the active site brings molecules together, similar to an adsorption site. However a particle immersed within a liquid is already surrounded by water molecules (Fig. 3b). In fact, nascent ice structures are associated with low-density regions within the liquid (Bullock and Molinero, 2013). Thus in the classical view the active site should be able to “pull molecules apart” instead of bringing them together. This creates a conceptual problem. To locally reduce ΔGhet active sites should be able to permanently create low-density regions within the liquid, which would require a large amount of energy. In other words, active sites would have the unusual property of creating a thermodynamic barrier maintaining their surrounding water in a non-equilibrium state. Such situation is unlikely in immersion freezing.
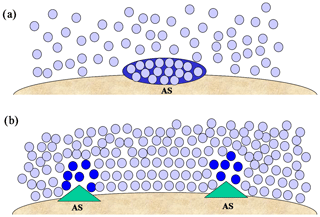
Figure 3Different representations of immersion freezing. (a) an ice germ (dark blue) forming on an active site (AS) by random collision of water molecules (light blue). (b) low-density regions (dark blue) forming in the vicinity of active sites within a dense liquid phase (light blue).
The concept of a local nucleation rate also presents some difficulties. In the strict sense Jhet is the velocity with which the size distribution of molecular clusters in an equilibrium population crosses the critical size (Kashchiev, 2000; Seinfeld and Pandis, 1998). In immersion freezing the domain of such a distribution is the whole volume of the droplet. Thus only a single value of Jhet can be defined for a continuous liquid phase, independently of where the actual nucleation process is occurring, since no permanent spatial gradients of T or concentration exist within equilibrium systems. Having otherwise implies that parts of the system would need to be maintained in a non-equilibrium state, having their own cluster size distribution. This requires the presence of non-permeable barriers within the liquid, a condition not encountered in immersion freezing. Similarly, the characteristic temperature of an active site is an unmeasurable quantity, since a system in equilibrium has the same temperature everywhere. Hence it would be impossible to distinguish whether the particle as a whole or only the active site must reach a certain temperature before nucleation takes place.
These difficulties can be reconciled if, instead of promoting nucleation through a thermodynamic mechanism, active sites provide a kinetic advantage to ice nucleation. A way in which this can be visualized is shown in Fig. 3b. The vicinal water is in equilibrium with the particle and exhibits a larger degree of ordering near the interface. Since in immersion freezing the formation of ice in the liquid depends on molecular rearrangement, the active site should produce a transient structural transformation that allows the propagation of ice. These sites would be characterized by defects where templating is not efficient, allowing greater molecular movement, hence facilitating restructuring. Their presence is guaranteed, since particles are never uniform at the molecular scale. In this view active sites create ice by promoting fluctuation instead of by locking water molecules in strict configurations. It implies that for uniform systems (e.g., a single droplet with a single particle) ΔGhet depends on the equilibrium between the particle and the vicinal water, and active sites enhance fluctuation around specific locations. This obviates the need for the hypothesis of a well-defined characteristic temperature for each active site. It, however, does not mean that active sites are transient. They are permanent features of the particle and should have a reproducible behavior, inducing ice nucleation around the same place in repeated experiments (Kiselev et al., 2017).
Within the framework presented above, there can only be one Jhet defined in the droplet volume. The presence of active sites introduces variability in J0 instead of ΔGhet. The latter is determined by the thermodynamic equilibrium between the particle and its vicinal water. Although the theory presented here does not account for internal gradients in the droplet–particle system, in practice it is likely that the observed Jhet corresponds to the site promoting the largest density fluctuations. Variability in Jhet would be introduced by fluctuation in the cluster size distribution in the liquid and from the multiplicity of active sites in the particle population. In this sense the proposed view is purely stochastic.
3.1 Ice nucleation regimes
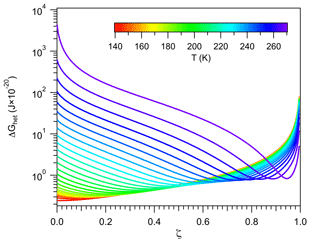
A consequence of the linkage between the properties of vicinal water and ΔGhet is the existence of distinct nucleation regimes. This was mentioned in Sect. 2.4.1, and here it is explored in detail. Recall from Fig. 2 that for a given temperature, ΔGhet passes by a minimum defined by the condition . Figure 4b depicts a similar behavior when varying T. It shows that for a given ζ there is a temperature Ts at which ΔGhet is minimum. For T>Ts, ΔGhet increases with increasing T because nhet increases (Fig. 4a). This is the typical behavior predicted by the classical model (Khvorostyanov and Curry, 2005), hence such regime will be termed “germ-forming”, since ΔGhet is determined by the formation of the ice–liquid interface.
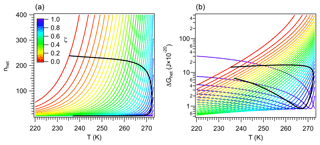
Figure 4Critical germ size (a) and work of heterogeneous ice nucleation (b) for different values of ζ (color). Black lines correspond to constant Jhet=106 m−2 s−1.
A different behavior is found for T<Ts, where ΔGhet decreases with increasing T. In this regime nhet remains almost constant at very low values, ΔGhet is small and results mostly from the dissipation of work. Ice nucleation is not limited by the formation of the ice–liquid interface but rather by the propagation of small fluctuations in the vicinity of preformed ice-like regions. Therefore it is controlled by the diffusion of water molecules to such regions rather than by ΔGhet. This is akin to a spinodal decomposition process (Cahn and Hilliard, 1958) and will be termed “spinodal ice nucleation”. It is, however, not truly spinodal decomposition, since it requires a finite, albeit small, amount of work to occur.
Since for each value of ζ there is a minimum in ΔGhet (Fig. 4), theoretically all INPs are capable of nucleating ice in both regimes. In practice spinodal ice nucleation would only occur if Ts lies within the 233 K K range, where immersion freezing occurs. For example, for ζ=0.1, Fig. 4b shows that the minimum in ΔGhet occurs at T<220 K. Since homogeneous ice nucleation should occur above this temperature, INPs characterized by ζ=0.1 will not exhibit spinodal ice nucleation. These particles always nucleate ice in the classical germ-forming regime (T>Ts). The situation is, however, different for ζ=0.9, since Ts≈270 K. These INPs are capable of nucleating ice in both the spinodal (T<Ts) and the germ-forming (T>Ts) regimes. For the spinodal regime, ΔGhet is low and decreases slightly with increasing T, indicating that the thermodynamic barrier to nucleation is virtually removed. Ice formation is therefore almost entirely controlled by kinetics.
The existence of the spinodal nucleation regime signals the possibility of an interesting behavior in freezing experiments, where the same ΔGhet may correspond to two very different INPs. To show this the values of ΔGhet and nhet corresponding to Jhet=106 m−2 s−1 are depicted in Fig. 4 with black lines. These lines form semi-closed curves when plotted against temperature indicating that the same ΔGhet may correspond to two different values of ζ. The upper branch (with high ΔGhet) corresponds to the germ-forming regime and the lower branch to the spinodal regime. This picture may be convoluted by the fact that high ζ also implies strong kinetic limitations during ice nucleation and is further discussed in Sect. 3.3.
3.2 Pre-exponential factor
Kinetic effects on ice nucleation are typically analyzed in terms of the pre-exponential factor, which is proportional to in the form
J0 expresses the normalized flux of water molecules to the ice germ, corrected by Z. Figure 5 shows J0 calculated using Eqs. (58) and (59). Results from CNT (Eq. 6) are also shown. In general J0 varies with T and ζ. The sensitivity of J0 to T is determined by D∞ (Barahona, 2015), with J0 increasing with T, since water molecules increase their mobility. Also, at higher T, less work is dissipated during interface transfer. These effects dominate the variation in J0 for ζ<0.5, suggesting that the particle has a limited effect on the mobility of vicinal water. Ice nucleation around these particles would be reasonably well described by assuming a negligible effect of the particle on J0, as done in CNT. This is evidenced by the CNT-derived values for and , which represent particles with high and low particle–ice affinity, respectively, and correspond to the range of expected variability in CNT. The and ζ∼0 lines in Fig. 5 are within 1 order of magnitude of each other and are in agreement with homogeneous nucleation results (Barahona, 2015). The line is also close to the ζ∼0.5 curve. In both cases J0 increases by about 2 orders of magnitude between 220 K and 273 K and decreases by about 2 orders of magnitude from ζ=0.0 to ζ=0.5, or from to in CNT. This reflects the effect of variation in Z on J0.
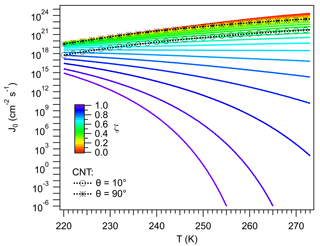
Figure 5Pre-exponential factor. Colored lines indicate different values of ζ. Black lines correspond to results calculated using CNT for different values of the contact angle, θ.
The behavior of J0 for ζ>0.5 dramatically differs from CNT. For ζ>0.5, and particularly for ζ>0.8, J0 decreases strongly with increasing T. This is because as ζ→1 and T→273 K, the driving force for interfacial transfer, i.e., the separation of μvc from thermodynamic equilibrium, vanishes. As the system moves near these conditions D becomes very small. This is the result of the high IL fraction of the vicinal water limiting the number of configurations available to form cooperative regions, required to induce water mobility (Sect. 2.5.1). Such behavior cannot be reproduced by CNT, since no explicit dependency of D on the properties of the vicinal layer is accounted for. For ζ>0.99 J0 decreases by more than 30 orders of magnitude from 220 K to 273K; molecular transport nearly stops. Ice nucleation may not be possible at such an extreme, despite the fact that these particles very efficiently reduce ΔGhet (Fig. 4); water may remain in the liquid state at very low temperatures. Such an effect has been experimentally observed in some biological systems (Wolfe et al., 2002).
3.3 Nucleation rate
The interplay between kinetics and thermodynamics determines the complex behavior of Jhet in immersion ice nucleation. Particles highly efficient at decreasing ΔGhet also decrease the rate of interfacial diffusion to the point where they may effectively prevent ice nucleation. On the other hand, INPs with low ζ do not significantly affect J0 but have a limited effect on ΔGhet. This is confounded with the presence of two thermodynamic nucleation regimes, one in which ΔGhet may be large and increases with T (“germ-forming”), and another in which ΔGhet is very small and decreases as T increases (“spinodal nucleation”). This picture can be simplified, since within the range K, where immersion freezing is relevant for atmospheric conditions, INPs with ζ>0.7 are, at the same time, more likely to nucleate ice in the spinodal regime and to exhibit strong kinetic limitations. Similarly for ζ<0.6 the transition to spinodal nucleation occurs below 233 K (Fig. 2). These INPs tend to nucleate ice in the germ-forming regime without significantly affecting J0. Thus the thermodynamic regimes introduced in Sect. 3.1 loosely correspond to kinetic regimes. Roughly, ice nucleation in the spinodal regime is controlled by kinetics, and in the germ-forming regime, it is controlled by thermodynamics. This is a useful approximation, but it should be used with caution. Even in the germ-forming regime the particle affects the kinetics of ice–liquid interfacial transfer to some extent. Similarly, in the spinodal regime ΔGhet is small, but finite.
Figure 6 shows the behavior of Jhet as T increases for different values of ζ. Jhet in the germ-forming regime resembles the behavior predicted by CNT. Jhet increases steeply with decreasing T and increasing ζ. Similarly for CNT, Jhet increases for decreasing T and θ. This is characteristic of the thermodynamic control on Jhet, where ΔGhet and are large (Fig. 4), and J0 is relatively unaffected by the particle. In this regime it is always possible to find a contact angle (typically between 10 and 100∘) that results in close agreement of Jhet between CNT and NNF predictions (Fig. 6), particularly for , which covers most values of atmospheric interest. This is also true for aw=0.9 (Fig. 6), although the approximation to the equilibrium temperature signals a steeper behavior in CNT peaking at higher values than NNF. Since is large, Jhet may show threshold behavior, characteristic of ice nucleation mediated by some dust species like Chlorite and Montmorillonite (Atkinson et al., 2013; Hoose and Möhler, 2012; Murray et al., 2012).
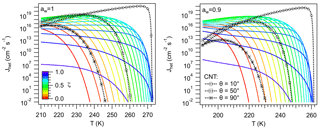
Figure 6Ice nucleation rate calculated using Eq. (61) for different values of ζ (color). Black lines were calculated using CNT for different values of the contact angle, θ.
There is, however, no value of θ that would lead to overlap between CNT and NNF for ζ>0.7. These conditions largely correspond to spinodal ice nucleation. Jhet is kinetically controlled, since ΔGhet is small, and J0 varies widely with T (Fig. 5). As in the germ-forming regime Jhet also reaches significant values but increases more slowly with decreasing T (Fig. 6). Higher ζ leads to Jhet becoming significant at higher T. But unlike in the germ-forming case, curves with higher ζ tend to plateau at progressively lower values of Jhet, since they become kinetically limited by their approximation to the thermodynamic equilibrium. For ζ∼0.7 some of the curves of Fig. 6 also display germ-forming behavior at high T and are characterized by a sudden decrease in as T decreases. The sudden change of slope corresponds to the region around the minimum ΔGhet (Fig. 4) and signals the transition from germ-forming to spinodal ice nucleation. Such behavior has been observed in some INPs of bacterial origin (Murray et al., 2012).
Figure 6 also indicates that nucleation regimes cannot be assigned based on the values of Jhet or on the observed freezing temperature, Tf. In both regimes, Jhet may reach substantial values, hence Tf may cover the entire range K. What is striking is that Jhet curves with ζ>0.7 tend to cross those with ζ<0.7. This means that two INPs characterized by very different ζ can have the same freezing temperature. This result thus challenges the common notion that INPs with higher freezing temperatures are intrinsically more active at nucleating ice, or in other words, that by measuring Tf alone, it is possible to characterize the freezing properties of a given material. In reality, to discern whether the observed Tf corresponds to a good (in the thermodynamic sense) INP acting in the spinodal regime or a less active INP acting in the germ-forming regime, it is necessary to measure along with Tf.
3.4 Application to the water activity-based nucleation rate
If a droplet is in equilibrium with its environment then aw is a function of the relative humidity. Thus the relationship between aw and the freezing temperature, Tf, conveys important information about the potential of a particle to catalyze the formation of ice and can be used to generate parameterizations of immersion ice nucleation for cloud models (Barahona and Nenes, 2009; Koop and Zobrist, 2009; Kärcher and Lohmann, 2003). A widely used class of parameterizations is based on the so-called water activity criterion (Koop and Zobrist, 2009; Koop et al., 2000), the condition that for a given material the water activity at which heterogeneous ice nucleation is observed, aw,het, is related by a constant to aw,eq (Koop and Zobrist, 2009; Koop et al., 2000). Here it is shown that the two-state thermodynamic model proposed in Sect. 2.3 implies the water activity criterion as a purely thermodynamic constraint to freezing.
3.4.1 Water activity shift
By definition the thermodynamic path shown in Fig. 1 operates between two equilibrium states. The relation between ΔGhet and ΔGhom is therefore independent of the way the system reaches aw,eff. In the absence of any kinetic limitations to the germ growth, Eq. (35) also represents a direct relationship between Jhom and Jhet. (Knopf and Alpert, 2013; Koop and Zobrist, 2009; Kärcher and Lohmann, 2003; Marcolli et al., 2007). Thus one can imagine two separate experiments in which the environmental conditions are set to either aw or aw,eff, the former resulting in heterogeneous freezing and the latter in homogeneous ice nucleation. Under these conditions Eq. (34) implies that when heterogeneous ice nucleation is observed at there is a corresponding homogeneous process that would occur at . Thus we can write an equivalent expression to Eq. (34), but in terms of aw,het and aw,hom, in the form
Eq. (63) can be rewritten as
Subtracting ln(aw,eq) from each side of Eq. (64) gives
Using the approximation for x∼1, Eq. (65) can be linearized in the form
where and are the homogeneous and heterogeneous water activity shifts, respectively. Δaw,hom has been found to be approximately constant for a wide range of solutes (Koop et al., 2000); therefore Eq. (66) suggests that Δaw,het should be approximately constant, since Λmix∼0.02 and only depends on T. Thus, the two-state model presented in Sect. 2.3 implies the so-called water activity criterion (Koop et al., 2000) for heterogeneous ice nucleation, giving support to the hypothesis that increasing order near the particle surface drives ice nucleation.
Equations (63) to (66) are fundamental thermodynamic relationships of the system and can be used to analyze the effect of the immersed particle on ice formation independently of kinetic effects. To do so aw,hom must be determined entirely by thermodynamics. This is because if aw,hom is defined at some Jhom threshold then it (and by extension aw,het) would also depend on the freezing kinetics. Fortunately, a thermodynamic definition of aw,hom has been achieved by Baker and Baker (2004). The authors showed that, on average, freezing occurs below the temperature at which the compressibility of water reaches a maximum. At this point density fluctuations are wide enough to allow for structural transformations that facilitate the formation of ice-like regions within the droplet volume. Such a criterion does not depend on measured freezing rates and can be extended to the freezing of water solutions, coinciding with the results of Koop et al. (2000). Bullock and Molinero (2013) also derived a pure thermodynamic criterion for aw,hom using the equilibrium between low-density regions and the bulk solution. Within these frameworks aw,hom can be defined without reference to a Jhom threshold. By extension, Eq. (64) guarantees that aw,het can be determined entirely by the thermodynamic properties of the system.
Equation (64) also implies that for a given aw,hom there is a temperature for which , referred as the “thermodynamic freezing temperature”, Tft. Formally, Tft represents the solution of
or in the linearized form,
Since Δaw,hom is considered a thermodynamic property of the system (Baker and Baker, 2004), Tft does not depend on the freezing kinetics. Thus Tft can be interpreted as the highest temperature where it is likely to observe ice nucleation for a given thermodynamic state (determined by aw, ζ and the system pressure).
Figure 7 shows the Tft–aw relationship defined by Eq. (67), calculated using (Baker and Baker, 2004; Barahona, 2014; Koop et al., 2000). As expected, the figure resembles experimental results found by several authors (Alpert et al., 2011; Knopf and Alpert, 2013; Koop and Zobrist, 2009; Zobrist et al., 2008; Zuberi et al., 2002), where curves for ζ>0 align with constant water activity shifts to aw,eq. To make this evident, lines were drawn using constant values of and 0.20, which coincide with lines corresponding to and 0.7, respectively. This shows that Eq. (66) is a good approximation to Eq. (63) and constitutes a theoretical derivation of the water activity criterion. The fact that such behavior can be reproduced by Eq. (63) validates the regular solution approximation used in Sect. 2.3 and supports the idea that the effect of the immersed particle on ice nucleation can be explained as a relative increase in the ice-like character of the vicinal water.
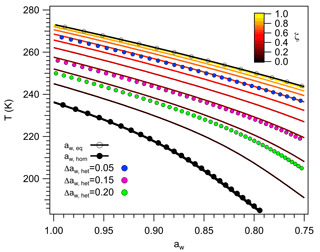
Figure 7Thermodynamic freezing temperature as a function of water activity. Colored lines correspond to for different values of ζ. Also shown are the water activities at equilibrium and at the homogeneous freezing threshold, aw,eq and aw,hom, respectively, and lines drawn applying constant water activity shifts, Δaw,het, of 0.05, 0.15 and 0.20.
It must be emphasized that Tft only establishes the potential of an INP to induce freezing at , regardless of whether a measurable Jhet can be experimentally realized. Physically, it is plausible that as the particle increases the ice-like character of the vicinal water, it also increases the probability of wide density fluctuations. As a result low-density regions, wide enough to accommodate the ice gem, exist at higher T than in homogeneous ice nucleation. Following the argument of Baker and Baker (2004) this would also imply that the compressibility of water near the particle reaches a maximum at higher T than in the bulk. More research however is needed to elucidate this point. The presence of a spinodal regime would also mean that the observed freezing temperature may differ from Tft, since at such a limit nucleation, it is no longer controlled by thermodynamics. This is illustrated in the next section.
3.4.2 Freezing by humic-like INPs
Δaw,het has been determined in several studies and has been used to predict and parameterize Jhet in atmospheric models (Knopf and Alpert, 2013; Zobrist et al., 2008). Thus it is useful in analyzing the conditions under which ζ (hence Jhet) can be estimated using measured Δaw,het values. Rearranging Eq. (66) we obtain
If Δaw,hom and Δaw,het are known, ζ can be estimated iteratively by solving Eq. (69). Note that Λmix is temperature dependent (Eq. 34), implying a slight dependency of ζ on T when Δaw,het is constant. However since Λmix is also typically small, ζ is almost equal to .
To test Eq. (69) the data for leonardite (LEO) and Pahokee peat (PP) particles (humic-like substances) obtained by Rigg et al. (2013) are used. The authors reported for LEO and for PP. These values are assumed to be independent of aw and T, with an experimental error in Δaw,het of 0.025. The average Jhet obtained from different samples and from repeated freezing and melting experiments for both materials is depicted in Fig. 8. Applying Eq. (69) over the T=210 K–250 K range and using results in for LEO and for PP. Within this temperature range these values correspond to the germ-forming regime, hence Jhet is thermodynamically controlled. A comparison against the experimentally determined Jhet for three different values of aw is shown in Fig. 8. Within the margin of error there is a reasonable agreement between the modeled and the experimental Jhet.
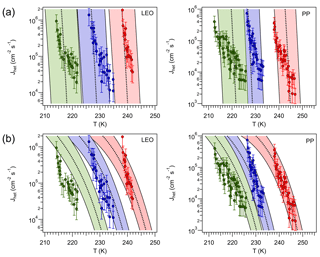
Figure 8(a) Heterogeneous ice nucleation rate calculated using a constant shift in aw (black, dotted, lines) for leonardite (LEO, ) and Pahokee peat (PP, ). Red, blue and green colors correspond to aw equal to 1.0, 0.931 and 0.872, respectively, for LEO, and 1.0, 0.901 and 0.862 for PP. Shaded area corresponds to . Markers correspond to experimental measurements reported by Rigg et al. (2013); error bars represent an order of magnitude deviation from the reported value. (b): Jhet calculated for constant ζ=0.949 for LEO and ζ=0.952 for PP. The shaded area corresponds to aw±0.01 and ζ±0.0015.
The top panels of Figure 8, however, reveal that even if Jhet becomes significant around the values predicted by Eq. (69), is overestimated, particularly for PP. This may indicate that these INPs nucleate ice in the spinodal regime. To test this hypothesis Jhet was fitted to the reported measurements by varying ζ within the range where spinodal nucleation would be dominant. To avoid agreement by design a single ζ was used for all experiments for each species resulting in ζ=0.949 for PP and ζ=0.952 for LEO (Fig. 8, bottom panels). For PP, Jhet and agree better with the experimental values, whereas for LEO the agreement improves at high T but worsens at low T. In this regime Jhet seems to be slightly overestimated by the theory at the lowest aw tested. This may be due to small uncertainties in aw that play a large role in Jhet (for example, the assumption of a T-independent aw; Alpert et al., 2011). There is the possibility that the humic acid present in PP may slightly dissolve during the experiments (Daniel Knopf, personal communication, 2017), which would impact not only aw but also may modify the composition of the particles, hence ζ.
The exercise above suggests that ice nucleation in PP may follow a spinodal mechanism. Using a single value of Δaw,het to predict ζ, as expressed mathematically by Eq. (69), seems to work for LEO. Since Eq. (69) represents a thermodynamic relation between Δaw,hom and Δaw,het, it is expected to work well when nucleation is thermodynamically controlled, i.e., the germ-forming regime. However it may fail for spinodal ice nucleation, since it does not consider the effect of the particle on J0. Δaw,het however carries important information about Jhet (Knopf and Alpert, 2013), but for spinodal ice nucleation, the relationship between Δaw,het and ζ must be more complex than predicted by Eq. (69), since kinetic limitations play a significant role. Figure 8 also shows that similar Tf can be obtained by either high or low ζ. The particular regime in which an INP nucleates ice determines , hence the sensitivity of the droplet freezing rate to the particle size and to the cooling rate.
3.5 Limitations
It is important to analyze the effect of several assumptions introduced in Sect. 2 on the theory presented here. One of the limitations of the approach used in deriving Eq. (61) is that it employs macroscale thermodynamics in the formulation of the work of nucleation. The effect of this assumption is, however, minimized in several ways. First, unlike frameworks based on the interfacial tension, NNF is much more robust to changes in ice germ size, since the product ΓwsΔhf remains constant (Sect. 2.4). Second, in the spinodal regime ΔGhet is independent of nhet, and only for T>268 K and in the germ-forming regime, the approach presented here may lead to uncertainty (Sect. 3.1). Thus Eq. (61) remains valid for most atmospheric conditions, although caution must be taken when Tf>268 K. Alternatively the framework presented here could be extended to account explicitly for the effect of size on Δhf and Γw (Zhang et al., 1999).
Further improvement could be achieved by implementing a more sophisticated equation of state of the vicinal water. Here a two-state assumption has been used, such that μvc is a linear combination of ice-like and liquid-like fractions. Such approximation has been used with success before (Etzler, 1983; Holten et al., 2013). However it is known that the structure of supercooled water represents an average of several distinct configurations (Stanley and Teixeira, 1980). These are, in principle, accounted for in the proposed approach, since ζ represents a relative, not an absolute, increase in the IL fraction. However there is no guarantee that such an increase can be linearly mapped in the way described in Sect. 2. Fortunately this would only mean, in practice, that the value of ζ for a given material is linked to the particular form of the equation of state used to describe the vicinal water.
Equation (61) is also blind to the surface properties of the immersed particle. The implicit assumption is that the effect of surface composition, charge, hydrophilicity and roughness on Jhet can be parameterized as a function of ζ. The example shown in Sect. 3.4 suggests that this is indeed the case. Making such relations explicit must, however, lie at the center of future development of the proposed approach. Similarly, a heuristic approach was used to study the effect of irreversibility on the nucleation work. This can be improved substantially by making use of a generalized Gibbs approach (Schmelzer et al., 2006), which unfortunately may also increase the number of free parameters in the model. None of these limitations is expected to change the conclusions of this study, however they may affect the values of ζ fitted when analyzing experimental data. The approach proposed here, however, has the advantage of being a simple, one-parameter approximation that can be easily implemented in cloud models.
Immersion freezing is a fundamental cloud process, and its correct representation in atmospheric models is critical for accurate climate and weather predictions. Current theories rely on a view that mimics ice formation from the vapor, neglecting several interactions unique to the liquid. This work develops, for the first time, a comprehensive approach to account for such interactions. The ice nucleation activity of immersed particles is linked to their effect on the vicinal water. It is shown that the same mechanism that lowers the thermodynamic barrier for ice nucleation also tends to decrease the mobility of water molecules, hence limiting interfacial transfer and ice germ growth. The role of the immersed particle in ice nucleation can be understood as increasing order in the adjacent water, facilitating the formation of ice-like structures. Thus, instead of being purely driven by thermodynamics, heterogeneous ice nucleation in the liquid phase is a process determined by the competition between thermodynamic and kinetic constraints to the formation and propagation of ice.
In the new approach the properties of vicinal water are approximated using a regular solution between high and low-density regions, with composition defined by an material specific parameter, ζ, which acts as a “templating factor” for ice nucleation. This results on an identity between the homogeneous and the heterogeneous work of nucleation (Eq. 35), implying that by knowing an expression for ΔGhom, ΔGhet can be readily written. This is advantageous, as homogeneous ice nucleation is far better understood than immersion ice nucleation and, because it avoids a mechanistic description of the complex interaction between the particle, the ice and the liquid. To describe ΔGhom the NNF framework (Barahona, 2014) was employed. This approach was extended to include non-equilibrium dissipation effects.
A model to describe the effect of the immersed particle on the mobility of water molecules, hence on the kinetics of immersion freezing, was also developed. This model builds upon an expression for the interfacial diffusion flux that accounts for the work required for water molecules to accommodate in an ice-like manner during interface transfer. Here this expression is extended to account for the effect of the particle on the molecular flux to the ice germ. It was shown that J0 strongly decreases as the system moves towards thermodynamic equilibrium.
The model presented here suggests the existence of a spinodal regime in ice nucleation where a pair of molecules with orientation similar to that of bulk ice may be enough to trigger freezing. Ice nucleation in the spinodal regime requires a highly efficient templating effect by the particle, however also tends to be strongly limited by the kinetics of the ice–liquid interfacial transfer. Compared to the classical germ-forming regime, nucleation by a spinodal mechanism is much more limited by diffusion and exhibits a more moderate increase in Jhet as temperature decreases. The existence of two nucleation regimes and the strong kinetic limitations occurring in efficient INPs imply that the freezing temperature is an ambiguous measure of ice nucleation activity. This is because, for a given T, two INPs characterized by different ζ may have the same Jhet, although with very different sensitivity to surface area and cooling rate.
The relationship between the measured shift in water activity Δaw,het and ζ was analyzed. It was shown that the proposed model leads directly to the derivation of the so-called water activity criterion for heterogeneous ice nucleation. The concept of “thermodynamic freezing temperature” was introduced and defined as the highest temperature at which it is likely to observe ice nucleation for a given thermodynamic state. Tft is useful in analyzing how changes in the thermodynamic environment around the droplet affect ice nucleation, independently of the freezing kinetics.
The theory presented here was tested using data for humic-like substances. It was found that assuming a fixed water activity shift to predict Jhet could be appropriate for low ζ, as found in leonardite (the germ-forming regime); however this may lead to overprediction of for the high ζ characterizing Pahokee peat INPs. This is because the water activity criterion represents a thermodynamic relation between aw and Tf but does not account for kinetic limitations, which may be significant in spinodal ice nucleation.
Immersion freezing research has seen a resurgence during the last decade (DeMott et al., 2011). A wealth of data is now available to test theories and new approaches to describe ice formation in atmospheric models. To do so effectively, it is necessary to develop models that realistically capture the complexities of the liquid phase. Further development of the approach presented here will look to better describe the nonreversible aspects of nucleation as well as to establish a more complete description of the properties of the vicinal water. The application to the freezing of atmospheric aerosol requires the definition of the ice nucleation spectrum, which will be pursued in a future work. Nevertheless, the present study constitutes, for first the time, an approximation to the modeling of ice nucleation that links the modifications of the properties of vicinal water by immersed particles with their ice nucleation ability. The approach presented here may help with expanding our understanding of immersion ice nucleation and facilitating the interpretation of experimental data in situations where current models fall short. The application of these ideas in cloud models will allow for the elucidation of the conditions under which different nucleation regimes occur in the atmosphere.
Supplementary material includes data used in generating Figs. 4–6. Fortran subroutines with the full implementation of the theory are available upon request.
| a0 | Cross-sectional area of a water molecule, , m2 |
| Aw | Phenomenological interaction parameter |
| aw | Activity of water |
| aw,eff | Effective water activity |
| aw,eq | Equilibrium aw between bulk liquid and ice (Koop and Zobrist, 2009) |
| aw,het | Thermodynamic freezing threshold for heterogeneous ice nucleation |
| aw,hom | Thermodynamic freezing threshold for homogeneous ice nucleation |
| C0 | Monomer concentration, m−2 |
| E,T0 | Parameters of the Vogel–Fulcher–Tammann equation defining D∞, 892 and 118 K(Smith and Kay, 1999) |
| D | Diffusion coefficient for interface transfer, m2 s−1 |
| D∞ | Self-diffusion coefficient of bulk water (Smith and Kay, 1999), m2 s−1 |
| D0 | Fitting parameter, m2 s−1(Smith and Kay, 1999) |
| d0 | Molecular diameter of water, , m |
| Impingement factor for heterogeneous ice nucleation, s−1 | |
| Impingement factor for homogeneous ice nucleation, s−1 | |
| G | Gibbs free energy, J |
| h | Planck's constant, J s |
| J0 | Pre-exponential factor |
| Jhet | Heterogeneous nucleation rate, |
| kB | Boltzmann constant, J K−1 |
| N | Number of clustering molecules in LL and IL regions, 6 (Holten et al., 2013) |
| n | Number of molecules in an ice cluster |
| n* | Critical germ size |
| nhet | Critical germ size for heterogeneous ice nucleation |
| nhom | Critical germ size for homogeneous ice nucleation |
| nt | Number of formation paths of the transient state, 16 (Barahona, 2015) |
| ps,w, ps,i | Liquid water and ice saturation vapor pressure, respectively, Pa(Murphy and Koop, 2005) |
| s | Geometric constant of the ice lattice, 1.105 molec1∕3(Barahona, 2014) |
| Si | Saturation ratio with respect to ice |
| Sc,0 | Configuration entropy of water* |
| Sc | Configuration entropy of vicinal water |
| T | Temperature, K |
| Tc | Critical separation temperature, 211.473 K |
| vw | Molecular volume of water in ice (Zobrist et al., 2007), m−3 |
| vw,0 | Molecular volume of water at 273.15 K |
| Average transition probability in water | |
| Wdiss | Work dissipated during cluster formation, J |
| Wd | Work dissipated during interface transfer, J |
| Z | Zeldovich factor |
| Δaw,het | aw,het − aw,eq |
| Δaw,hom | aw,hom − aw,eq, 0.304 (Barahona, 2014; Koop et al., 2000) |
| ΔG | Work of cluster formation, J |
| ΔGact | Activation energy for ice nucleation, J |
| ΔGhom | Nucleation work for homogeneous ice nucleation, J |
| ΔGhet | Nucleation work for heterogeneous ice nucleation, J |
| Δhf | Heat of solidification of water, J mol−1(Barahona et al., 2014; Johari et al., 1994) |
| Δμs | Excess free energy of solidification of water, J |
| Δμi | Driving force for ice nucleation, J |
| Λmix | Dimensionless mixing parameter, defined in Eq. (30) |
| Φ | Energy of formation of the ice–liquid interface, molec1∕3 J |
| Γw | Molecular surface excess at the interface, 1.46 (Barahona et al., 2014; Spaepen, 1975) |
| μw, μs,μvc | Chemical potential of water, ice and vicinal water, respectively J |
| ρw, ρi | Bulk density of liquid water and ice, respectively, Kg m−3(Pruppacher and Klett, 1997) |
| σE | Dimensionless residual entropy |
| σiw | Ice–liquid interfacial energy J m−2(Barahona et al., 2014) |
| θ | Contact angle |
| ζ | Templating factor |
| Ωg | Ice germ surface area, m−2 |
| * From the data of Scala et al. (2000), the following fit was obtained: | |
| for T between 180 and 273 K. | |
The supplement related to this article is available online at: https://doi.org/10.5194/acp-18-17119-2018-supplement.
The author declares that there is no conflict of interest.
Donifan Barahona was supported by the NASA Modeling and Analysis Program,
grant: 16-MAP16-0085.
Edited by: Martina
Krämer
Reviewed by: three anonymous referees
Adam, G. and Gibbs, J. H.: On the temperature dependence of cooperative relaxation properties in glass-forming liquids, J. Chem. Phys., 43, 139–146, 1965. a, b
Alpert, P. A., Aller, J. Y., and Knopf, D. A.: Ice nucleation from aqueous NaCl droplets with and without marine diatoms, Atmos. Chem. Phys., 11, 5539–5555, https://doi.org/10.5194/acp-11-5539-2011, 2011. a, b, c
Anderson, D. M.: Ice nucleation and the substrate-ice interface, Nature, 216, 563–566, https://doi.org/10.1038/216563a0, 1967. a, b, c
Atkinson, J. D., Murray, B. J., Woodhouse, M. T., Whale, T. F., Baustian, K. J., Carslaw, K. S., Dobbie, S., O'sullivan, D., and Malkin, T. L.: The importance of feldspar for ice nucleation by mineral dust inmixed-phase clouds, Nature, 498, 355–358, https://doi.org/10.1038/nature12278, 2013. a
Baker, M. and Baker, M.: A new look at homogeneous freezing of water, Geophys. Res. Lett., 31, L19102, https://doi.org/10.1029/2004GL020483, 2004. a, b, c, d
Barahona, D.: Analysis of the effect of water activity on ice formation using a new thermodynamic framework, Atmos. Chem. Phys., 14, 7665–7680, https://doi.org/10.5194/acp-14-7665-2014, 2014. a, b, c, d, e, f, g, h, i, j, k, l, m
Barahona, D.: Thermodynamic derivation of the activation energy for ice nucleation, Atmos. Chem. Phys., 15, 13819–13831, https://doi.org/10.5194/acp-15-13819-2015, 2015. a, b, c, d, e, f, g, h, i, j
Barahona, D. and Nenes, A.: Parameterization of cirrus formation in large scale models: Homogeneous nucleation, J. Geophys. Res., 113, D11211, https://doi.org/10.1029/2007JD009355, 2008. a
Barahona, D. and Nenes, A.: Parameterizing the competition between homogeneous and heterogeneous freezing in cirrus cloud formation – monodisperse ice nuclei, Atmos. Chem. Phys., 9, 369–381, https://doi.org/10.5194/acp-9-369-2009, 2009. a, b
Barahona, D., Molod, A., Bacmeister, J., Nenes, A., Gettelman, A., Morrison, H., Phillips, V., and Eichmann, A.: Development of two-moment cloud microphysics for liquid and ice within the NASA Goddard Earth Observing System Model (GEOS-5), Geosci. Model Dev., 7, 1733–1766, https://doi.org/10.5194/gmd-7-1733-2014, 2014. a, b, c, d
Barahona, D., Molod, A., and Kalesse, H.: Direct estimation of the global distribution of vertical velocity within cirrus clouds, Sci. Rep. UK, 7, 6840, https://doi.org/10.1038/s41598-017-07038-6, 2017. a
Bellissent-Funel, M.-C.: Water near hydrophilic surfaces, J. Mol. Liquids, 96, 287–304, https://doi.org/10.1016/S0167-7322(01)00354-3, 2002. a
Bigg, E. K.: The formation of atmospheric ice crystals by the freezing of droplets, Q. J. Roy. Meteor. Soc., 79, 510–519, https://doi.org/10.1002/qj.49707934207, 1953. a
Black, S.: Simulating nucleation of molecular solids, P. R. Soc. A, 463, 2799–2811, 2007. a
Broadley, S. L., Murray, B. J., Herbert, R. J., Atkinson, J. D., Dobbie, S., Malkin, T. L., Condliffe, E., and Neve, L.: Immersion mode heterogeneous ice nucleation by an illite rich powder representative of atmospheric mineral dust, Atmos. Chem. Phys., 12, 287–307, https://doi.org/10.5194/acp-12-287-2012, 2012. a
Brukhno, A. V., Anwar, J., Davidchack, R., and Handel, R.: Challenges in molecular simulation of homogeneous ice nucleation, J. Phys.-Condens. Mat., 20, 494243, https://doi.org/10.1088/0953-8984/20/49/494243, 2008. a
Bullock, G. and Molinero, V.: Low-density liquid water is the mother of ice: on the relation between mesostructure, thermodynamics and ice crystallization in solutions, Faraday Discuss., https://doi.org/10.1039/C3FD00085K, 2013. a, b, c
Cahn, J. W.: Surface stress and the chemical equilibrium of small crystals–I. The case of the isotropic surface, Acta Metall. Mater, 28, 1333–1338, 1980. a, b, c
Cahn, J. W. and Hilliard, J. E.: Free energy of a nonuniform system. I. Interfacial free energy, J. Chem. Phys., 28, 258–267, 1958. a
Cooke, R. and Kuntz, I.: The properties of water in biological systems, Ann. Review Biophys. Bioeng., 3, 95–126, 1974. a
Cox, S. J., Kathmann, S. M., Slater, B., and Michaelides, A.: Molecular simulations of heterogeneous ice nucleation. I. Controlling ice nucleation through surface hydrophilicity, J. Chem. Phys, 142, 184704, https://doi.org/10.1063/1.4919714, 2015. a, b
Cziczo, D. J., Froyd, K. D., Hoose, C., Jensen, E. J., Diao, M., Zondlo, M. A., Smith, J. B., Twohy, C. H., and Murphy, D. M.: Clarifying the Dominant Sources and Mechanisms of Cirrus Cloud Formation, Science, 340, 1320–1324, 2013. a
De Gennes, P.-G.: Wetting: statics and dynamics, Rev. Modern Phys., 57, 827–863, https://doi.org/10.1103/RevModPhys.57.827, 1985. a
Debenedetti, P. G. and Stillinger, F. H.: Supercooled liquids and the glass transition, Nature, 410, 259–267, 2001. a, b
DeMott, P., Cziczo, D., Prenni, A., Murphy, D., Kreidenweis, S., Thompson, D., Borys, R., and Rogers, D.: Measurements of the concentration and composition of nuclei for cirrus formation, Proc. Natl. Acad. Sci. USA, 100, 14655–14660, 2003. a, b
DeMott, P. J., Prenni, A. J., Liu, X., Kreidenweis, S. M., Petters, M. D., Twohy, C. H., Richardson, M. S., Eidhammer, T., and Rogers, D. C.: Predicting global atmospheric ice nuclei distributions and their impacts on climate, Proc. Natl. Acad. Sci. USA, 107, 11217–11222, https://doi.org/10.1073/pnas.0910818107, 2010. a
DeMott, P. J., Möhler, O., Stetzer, O., Vali, G., Levin, Z., Petters, M. D., Murakami, M., Leisner, T., Bundke, U., Klein, H., et al.: Resurgence in ice nuclei measurement research, B. Am. Meteorol. Soc., 92, 1623–1635, 2011. a
Diehl, K. and Wurzler, S.: Heterogeneous drop freezing in the immersion mode: model calculations considering soluble and insoluble particles in the drops, J. Atmos. Sci., 61, 2063–2072, 2004. a
Drost-Hansen, W.: Structure of water near solid interfaces, Ind. & Eng. Chem., 61, 10–47, 1969. a, b, c, d
Espinosa, J., Sanz, E., Valeriani, C., and Vega, C.: Homogeneous ice nucleation evaluated for several water models, J. Chem. Phys., 141, 18C529, https://doi.org/10.1063/1.4897524, 2014. a
Etzler, F. M.: A statistical thermodynamic model for water near solid interfaces, J. Coll. Interf. Sci., 92, 43–56, 1983. a, b, c, d, e, f
Feibelman, P. J.: The first wetting layer on a solid, Physics today, 63, 34, https://doi.org/10.1063/1.3326987, 2010. a, b
Fitzner, M., Sosso, G. C., Cox, S. J., and Michaelides, A.: The Many Faces of Heterogeneous Ice Nucleation: Interplay Between Surface Morphology and Hydrophobicity, J. Am. Chem. Soc., 137, 13658–13669, https://doi.org/10.1021/jacs.5b08748, 2015. a
Fletcher, H.: On ice-crystal production by aerosol particles, J. Atmos. Sci., 16, 173–180, 1959. a
Gettelman, A., Liu, X., Barahona, D., Lohmann, U., and Chen, C.: Climate impacts of ice nucleation, J. Geophys. Res., 117, D20201, https://doi.org/10.1029/2012JD017950, 2012. a
Hiranuma, N., Augustin-Bauditz, S., Bingemer, H., Budke, C., Curtius, J., Danielczok, A., Diehl, K., Dreischmeier, K., Ebert, M., Frank, F., Hoffmann, N., Kandler, K., Kiselev, A., Koop, T., Leisner, T., Möhler, O., Nillius, B., Peckhaus, A., Rose, D., Weinbruch, S., Wex, H., Boose, Y., DeMott, P. J., Hader, J. D., Hill, T. C. J., Kanji, Z. A., Kulkarni, G., Levin, E. J. T., McCluskey, C. S., Murakami, M., Murray, B. J., Niedermeier, D., Petters, M. D., O'Sullivan, D., Saito, A., Schill, G. P., Tajiri, T., Tolbert, M. A., Welti, A., Whale, T. F., Wright, T. P., and Yamashita, K.: A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: a comparison of 17 ice nucleation measurement techniques, Atmos. Chem. Phys., 15, 2489–2518, https://doi.org/10.5194/acp-15-2489-2015, 2015. a
Holten, V., Limmer, D. T., Molinero, V., and Anisimov, M. A.: Nature of the anomalies in the supercooled liquid state of the mW model of water, J. Chem. Phys., 138, 174501, https://doi.org/10.1063/1.4802992, 2013. a, b, c, d, e, f, g, h
Hoose, C. and Möhler, O.: Heterogeneous ice nucleation on atmospheric aerosols: a review of results from laboratory experiments, Atmos. Chem. Phys., 12, 9817–9854, https://doi.org/10.5194/acp-12-9817-2012, 2012. a, b, c, d
Hoose, C., Kristjansson, J., Chen, J.-C., and Hazra, A.: A classical-theory-based parameterization of heterogeneous ice nucleation by mineral dust, soot, and biological particles in a global climate model, J. Atmos. Sci., 67, 2483–2503, https://doi.org/10.1175/2010JAS3425.1, 2010. a, b
Ickes, L., Welti, A., and Lohmann, U.: Classical nucleation theory of immersion freezing: sensitivity of contact angle schemes to thermodynamic and kinetic parameters, Atmos. Chem. Phys., 17, 1713–1739, https://doi.org/10.5194/acp-17-1713-2017, 2017. a, b
Johari, G., Fleissner, G., Hallbrucker, A., and Mayer, E.: Thermodynamic continuity between glassy and normal water, J. Phys. Chem., 98, 4719–4725, 1994. a
Johnston, J. C. and Molinero, V.: Crystallization, melting, and structure of water nanoparticles at atmospherically relevant temperatures, JACS, 134, 6650–6659, https://doi.org/10.1021/ja210878c, 2012. a, b
Kalikmanov, V. I. and van Dongen, M. E. H.: Self-consistent cluster approach to the homogeneous kinetic nucleation theory, Phys. Rev. E, 47, 3532–3539, https://doi.org/10.1103/PhysRevE.47.3532, 1993. a
Kärcher, B. and Lohmann, U.: A parameterization of cirrus cloud formation: Heterogeneous freezing, J. Geophys. Res., 108, 4402, https://doi.org/10.1029/2002JD003220, 2003. a, b
Kashchiev, D.: Nucleation: basic theory with applications, Butterworth Heinemann, Burlington, MA, USA, 2000. a, b, c, d, e, f, g, h, i, j
Khvorostyanov, V. and Curry, J.: The theory of ice nucleation by heterogeneous freezing of deliquescent mixed CCN. Part I: critical radius, energy and nucleation rate, J. Atmos. Sci., 61, 2676–2691, 2004. a, b, c
Khvorostyanov, V. and Curry, J.: The theory of ice nucleation by heterogeneous freezing of deliquescent mixed CCN. Part II: parcel model simulations, J. Atmos. Sci., 62, 261–285, 2005. a
Kiselev, A., Bachmann, F., Pedevilla, P., Cox, S. J., Michaelides, A., Gerthsen, D., and Leisner, T.: Active sites in heterogeneous ice nucleation – the example of K-rich feldspars, Science, 355, 367–371, https://doi.org/10.1126/science.aai8034, 2017. a, b
Knopf, D. A. and Alpert, P. A.: A water activity based model of heterogeneous ice nucleation kinetics for freezing of water and aqueous solution droplets, Faraday Discuss., 165, 513–534, 2013. a, b, c, d, e
Koop, T. and Zobrist, B.: Parameterizations for ice nucleation in biological and atmospheric systems, Phys. Chem. Chem. Phys., 11, 10839–10850, 2009. a, b, c, d, e, f
Koop, T., Luo, B., Tslas, A., and Peter, T.: Water activity as the determinant for homogeneous ice nucleation in aqueous solutions, Nature, 406, 611–614, 2000. a, b, c, d, e, f, g
Lance, S., Shupe, M. D., Feingold, G., Brock, C. A., Cozic, J., Holloway, J. S., Moore, R. H., Nenes, A., Schwarz, J. P., Spackman, J. R., Froyd, K. D., Murphy, D. M., Brioude, J., Cooper, O. R., Stohl, A., and Burkhart, J. F.: Cloud condensation nuclei as a modulator of ice processes in Arctic mixed-phase clouds, Atmos. Chem. Phys., 11, 8003–8015, https://doi.org/10.5194/acp-11-8003-2011, 2011. a
Li, K., Xu, S., Chen, J., Zhang, Q., Zhang, Y., Cui, D., Zhou, X., Wang, J., and Song, Y.: Viscosity of interfacial water regulates ice nucleation, App. Phys. Lett., 104, 101605, https://doi.org/10.1063/1.4868255, 2014. a
Lohmann, U. and Feichter, J.: Global indirect aerosol effects: a review, Atmos. Chem. Phys., 5, 715–737, https://doi.org/10.5194/acp-5-715-2005, 2005. a
Lupi, L., Hudait, A., and Molinero, V.: Heterogeneous Nucleation of Ice on Carbon Surfaces, J. Am. Chem. Soc., 136, 3156–3164, 2014. a, b, c
Marcolli, C., Gedamke, S., Peter, T., and Zobrist, B.: Efficiency of immersion mode ice nucleation on surrogates of mineral dust, Atmos. Chem. Phys., 7, 5081–5091, https://doi.org/10.5194/acp-7-5081-2007, 2007. a
Matsumoto, M., Saito, S., and Ohmine, I.: Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing, Nature, 416, 409–413, 2002. a
Meyers, M., DeMott, P., and Cotton, R.: New primary ice-nucleation parameterization in an explicit cloud model, J. Appl. Meteorol., 31, 708–721, 1992. a
Michaelides, A. and Morgenstern, K.: Ice nanoclusters at hydrophobic metal surfaces, Nat. Mater., 6, 597–601, https://doi.org/10.1038/nmat1940, 2007. a
Michot, L. J., Villiéras, F., François, M., Bihannic, I., Pelletier, M., and Cases, J.-M.: Water organisation at the solid–aqueous solution interface, C. R. Geosci., 334, 611–631, 2002. a, b, c
Murphy, D. and Koop, T.: Review of the vapour pressures of ice and supercooled water for atmospheric applications, Q. J. Roy. Meteor. Soc., 131, 1539–1565, 2005. a
Murray, B. J., O'sullivan, D., Atkinson, J. D., and Webb, M. E.: Ice nucleation by particles immersed in supercooled cloud droplets, Chem. Soc. Rev., 41, 6519–6554, https://doi.org/10.1039/C2CS35200A, 2012. a, b, c, d, e, f, g, h
Myhre, G., Shindell, D., Breon, F.-M., Collins, W., Fuglestvedt, J., Huang, J., Koch, D., Lamarque, J.-F., Lee, D., Mendoza, B., Nakajima, T., Robock, A., Stephens, G., Takemura, T., and Zhang, H.: Anthropogenic and Natural Radiative Forcing. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, book section 8, 659–740, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, https://doi.org/10.1017/CBO9781107415324.018, 2013. a
Niemand, M., Möhler, O., Vogel, B., Vogel, H., Hoose, C., Connolly, P., Klein, H., Bingemer, H., DeMott, P., Skrotzki, J., and Leisner, T.: A particle-surface-area-based parameterization of immersion freezing on desert dust particles, J. Atmos. Sci., 69, 3077–3092, https://doi.org/10.1175/JAS-D-11-0249.1, 2012. a
O, K.-T. and Wood, R.: Exploring an approximation for the homogeneous freezing temperature of water droplets, Atmos. Chem. Phys., 16, 7239–7249, https://doi.org/10.5194/acp-16-7239-2016, 2016. a
Pauling, L.: The structure and entropy of ice and of other crystals with some randomness of atomic arrangement, J. Am. Chem. Soc., 57, 2680–2684, 1935. a
Phillips, V. T., Demott, P. J., Andronache, C., Pratt, K. A., Prather, K. A., Subramanian, R., and Twohy, C.: Improvements to an Empirical Parameterization of Heterogeneous Ice Nucleation and its Comparison with Observations, J. Atmos. Sci., 70, 378–409, https://doi.org/10.1175/JAS-D-12-080.1, 2013. a
Prausnitz, J. M., Lichtenthaler, R. N., and de Azevedo, E. G.: Molecular thermodynamics of fluid-phase equilibria, Prentice Hall, Upper Saddle River, NJ, USA, 3rd edn., 1998. a, b, c
Pruppacher, H. and Klett, J.: Microphysics of clouds and precipitation, Kluwer Academic Publishers, Boston, MA, 2nd edn., 1997. a, b, c, d, e, f, g, h, i
Rigg, Y. J., Alpert, P. A., and Knopf, D. A.: Immersion freezing of water and aqueous ammonium sulfate droplets initiated by humic-like substances as a function of water activity, Atmos. Chem. Phys., 13, 6603–6622, https://doi.org/10.5194/acp-13-6603-2013, 2013. a, b, c
Rinnert, E., Carteret, C., Humbert, B., Fragneto-Cusani, G., Ramsay, J. D., Delville, A., Robert, J.-L., Bihannic, I., Pelletier, M., and Michot, L. J.: Hydration of a synthetic clay with tetrahedral charges: a multidisciplinary experimental and numerical study, J. Phys. Chem. B, 109, 23745–23759, 2005. a
Rusanov, A. I.: Surface thermodynamics revisited, Surf. Sc. Reports, 58, 111–239, 2005. a
Scala, A., Starr, F. W., La Nave, E., Sciortino, F., and Stanley, H. E.: Configurational entropy and diffusivity of supercooled water, Nature, 406, 166–169, 2000. a, b, c
Schmelzer, J. W., Boltachev, G. S., and Baidakov, V. G.: Classical and generalized Gibbs' approaches and the work of critical cluster formation in nucleation theory, J. Chem. Phys., 124, 194503, https://doi.org/10.1063/1.2196412, 2006. a
Seinfeld, J. H. and Pandis, S. N.: Atmospheric Chemistry and Physics, John Wiley and Sons, New York, NY, USA, 1998. a
Smith, R. S. and Kay, B. D.: The existence of supercooled liquid water at 150 K, Nature, 398, 788–791, 1999. a, b, c
Snyder, P. W., Lockett, M. R., Moustakas, D. T., and Whitesides, G. M.: Is it the shape of the cavity, or the shape of the water in the cavity?, The European Physical Journal Special Topics, 223, 853–891, https://doi.org/10.1140/epjst/e2013-01818-y, 2014. a, b
Spaepen, F.: A structural model for the solid-liquid interface in monatomic systems, Acta Metall. Mater, 23, 729–743, 1975. a, b, c
Stanley, H. E. and Teixeira, J.: Interpretation of the unusual behavior of H2O and D2O at low temperatures: tests of a percolation model, J. Chem. Phys., 73, 3404–3422, 1980. a, b
Tan, I., Storelvmo, T., and Zelinka, M. D.: Observational constraints on mixed-phase clouds imply higher climate sensitivity, Science, 352, 224–227, https://doi.org/10.1126/science.aad5300, 2016. a
Taylor, J. H. and Hale, B. N.: Monte Carlo simulations of water-ice layers on a model silver iodide substrate: A comparison with bulk ice systems, Physical Rev. B, 47, 9732, 1993. a
Turnbull, D. and Fisher, J. C.: Rate of nucleation in condensed systems, J. Chem. Phys., 17, 71–73, 1949. a
Vali, G.: Interpretation of freezing nucleation experiments: singular and stochastic; sites and surfaces, Atmos. Chem. Phys., 14, 5271–5294, https://doi.org/10.5194/acp-14-5271-2014, 2014. a
Vekilov, P. G.: The two-step mechanism of nucleation of crystals in solution, Nanoscale, 2, 2346–2357, https://doi.org/10.1039/C0NR00628A, 2010. a, b
Wang, J., Kalinichev, A. G., and Kirkpatrick, R. J.: Effects of substrate structure and composition on the structure, dynamics, and energetics of water at mineral surfaces: A molecular dynamics modeling study, Geochim. Cosmochim. Ac., 70, 562–582, https://doi.org/10.1016/j.gca.2005.10.006, 2006. a
Wang, Q., Zhao, L., Li, C., and Cao, Z.: The decisive role of free water in determining homogenous ice nucleation behavior of aqueous solutions, Sci. Rep. UK, 6, 26831, https://doi.org/10.1038/srep26831, 2016. a
Warne, M., Allan, N., and Cosgrove, T.: Computer simulation of water molecules at kaolinite and silica surfaces, Phys. Chem. Chem. Phys., 2, 3663–3668, 2000. a
Wiacek, A., Peter, T., and Lohmann, U.: The potential influence of Asian and African mineral dust on ice, mixed-phase and liquid water clouds, Atmos. Chem. Phys., 10, 8649–8667, https://doi.org/10.5194/acp-10-8649-2010, 2010. a, b
Wolfe, J., Bryant, G., and Koster, K. L.: What is' unfreezable water', how unfreezable is it and how much is there?, CryoLetters, 23, 157–166, 2002. a, b, c
Yu, C.-J., Evmenenko, G., Richter, A., Datta, A., Kmetko, J., and Dutta, P.: Order in molecular liquids near solid–liquid interfaces, App. Surface Sci., 182, 231–235, https://doi.org/10.1016/S0169-4332(01)00410-X, 2001. a, b, c
Zhang, Z., Lü, X., and Jiang, Q.: Finite size effect on melting enthalpy and melting entropy of nanocrystals, Physica B, 270, 249–254, https://doi.org/10.1016/S0921-4526(99)00199-4, 1999. a, b
Zheng, J.-m., Chin, W.-C., Khijniak, E., Khijniak Jr, E., and Pollack, G. H.: Surfaces and interfacial water: evidence that hydrophilic surfaces have long-range impact, Adv. Colloid Interface Sc., 127, 19–27, https://doi.org/10.1016/j.cis.2006.07.002, 2006. a
Zobrist, B., Koop, T., Luo, B., Marcolli, C., and Peter, T.: Heterogeneous ice nucleation rate coefficient of water droplets coated by a nonadecanol monolayer, J. Phys. Chem. C, 111, 2149–2155, 2007. a, b, c, d, e
Zobrist, B., Marcolli, C., Peter, T., and Koop, T.: Heterogeneous ice nucleation in aqueous solutions: the role of water activity, J. Phys. Chem. A, 112, 3965–3975, 2008. a, b
Zuberi, B., Bertram, A., Cassa, C., Molina, L., and Molina, M.: Heterogeneous nucleation of ice in (NH4)2SO4-H2O particles with mineral dust immersions, Geophys. Res. Lett., 29, 1504, https://doi.org/10.1029/2001GL014289, 2002. a